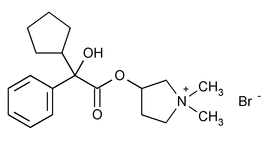
Glycopyrrolate
C19H28BrNO3 398.33
Pyrrolidinium, 3-[ (SR)-
(SR)- USP35(cyclopentylhydroxyphenylacetyl)oxy]-1,1-dimethyl-,
USP35(cyclopentylhydroxyphenylacetyl)oxy]-1,1-dimethyl-,  [RS-]
[RS-] USP35 bromide;
USP35 bromide;
 (RS)-[3-(SR)-Hydroxy-1,1-dimethylpyrrolidinium bromide]
(RS)-[3-(SR)-Hydroxy-1,1-dimethylpyrrolidinium bromide]  -cyclopentylmandelate
-cyclopentylmandelate USP35
USP35


 [596-51-0].
[596-51-0].
 The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay. USP35
USP35
 Buffer:
Prepare a solution of 1.0 g of anhydrous sodium sulfate and 200 mg of sodium 1-hexanesulfonate monohydrate in 650 mL of water. To this solution add 3.0 mL of 1 N sulfuric acid, and mix.
Buffer:
Prepare a solution of 1.0 g of anhydrous sodium sulfate and 200 mg of sodium 1-hexanesulfonate monohydrate in 650 mL of water. To this solution add 3.0 mL of 1 N sulfuric acid, and mix.
 • Ordinary Impurities
• Ordinary Impurities  466
466
 • Organic Impurities
• Organic Impurities
 USP35
USP35
 • Limit of Erythro Isomer
• Limit of Erythro Isomer
 USP35
USP35
 • Melting Range or Temperature, Class I
• Melting Range or Temperature, Class I  741
741 :
193
:
193 –198
–198 , but the range between beginning and end of melting does not exceed 2
, but the range between beginning and end of melting does not exceed 2 .
. USP35
USP35
 Store at room temperature.
Store at room temperature. USP35
USP35
 11
11
 USP Glycopyrrolate Related Compound A RS
USP Glycopyrrolate Related Compound A RS
5-Nitrobenzene-1,3-dicarboxylic acid.
C8H5NO6 211.13
 USP35
USP35
(glye'' koe pir' oh late).
Change to read:
C19H28BrNO3 398.33
Pyrrolidinium, 3-[
DEFINITION
Change to read:
Glycopyrrolate 
 USP35 contains NLT 98.0% and NMT
USP35 contains NLT 98.0% and NMT  102.0%
102.0% USP35 of C19H28BrNO3,
USP35 of C19H28BrNO3,  calculated on the dried basis.
calculated on the dried basis. USP35
USP35
IDENTIFICATION
Change to read:
• B.
• C. Identification Tests—General, Bromide  191
191
Sample solution:
25 mg/mL
Acceptance criteria:
Meets the requirements
ASSAY
Change to read:
• Procedure
Mobile phase:
Acetonitrile, methanol, and Buffer (20:15:65)
Standard solution:
0.1 mg/mL of USP Glycopyrrolate RS in Mobile phase
Sample solution:
0.1 mg/mL of Glycopyrrolate in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 222 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Column temperature:
40
Flow rate:
1.2 mL/min
Injection size:
50 µL
System suitability
Sample:
Standard solution
Suitability requirements
Tailing factor:
NMT 2.0
Relative standard deviation:
NMT 1.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of glycopyrrolate (C19H28BrNO3) in the portion of Glycopyrrolate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of Glycopyrrolate from the Sample solution |
| rS | = | = peak response of glycopyrrolate from the Standard solution |
| CS | = | = concentration of USP Glycopyrrolate RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Glycopyrrolate in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% on the dried basis USP35
USP35
IMPURITIES
• Residue on Ignition  281
281 :
NMT 0.3%
:
NMT 0.3%
Delete the following:
Standard solutions:
0.05, 0.25, 0.5, and 1.0 mg/mL in alcohol
Sample solution:
50 mg/mL in alcohol
Eluant:
A mixture of ethyl acetate, water, and anhydrous formic acid (74:16:10)
Adsorbent:
Chromatographic silica gel
Application volume:
5 µL
Visualization:
Dry the plate at 105 for 15 min, followed by Visualization Technique 3; then air-dry the developed plate at room temperature for 2 h.
for 15 min, followed by Visualization Technique 3; then air-dry the developed plate at room temperature for 2 h.
Acceptance criteria:
The intensity of any secondary spot of the Sample solution corresponds to NMT 0.5%, and the sum of the intensities of all secondary spots of the Sample solution corresponds to NMT 2.0%. USP35
USP35
Add the following:
Buffer:
Prepare a solution of 1.0 g of anhydrous sodium sulfate and 200 mg of sodium 1-hexanesulfonate monohydrate in 650 mL of water. To this solution add 3.0 mL of 1 N sulfuric acid, and mix.
Diluent:
Prepare a solution of 1.0 g of anhydrous sodium sulfate, 6.8 g of monobasic potassium phosphate, and 200 mg of sodium 1-hexanesulfonate monohydrate in 650 mL of water. To this solution add 3.0 mL of 1 N sulfuric acid, 150 mL of methanol, and 200 mL of acetonitrile, and mix. Adjust with phosphoric acid to a pH of 2.8.
Solution A:
Acetonitrile, methanol, and Buffer (20:15:65)
Solution B:
Acetonitrile, methanol, and Buffer (50:15:35)
Mobile phase:
See Table 1.
Table 1
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 100 | 0 |
| 10 | 100 | 0 |
| 25 | 10 | 90 |
| 35 | 10 | 90 |
| 37 | 100 | 0 |
| 45 | 100 | 0 |
Standard solution:
1.5 µg/mL each of USP Glycopyrrolate RS, USP Glycopyrrolate Related Compound A RS, USP Glycopyrrolate Related Compound B RS, and USP Glycopyrrolate Related Compound C RS in Diluent. Sonicate, if necessary, to facilitate dissolution.
Sample solution:
1.0 mg/mL of Glycopyrrolate in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 222 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Column temperature:
40
Flow rate:
1 mL/min
Injection size:
50 µL
System suitability
Sample:
Standard solution
Suitability requirements
Resolution:
NLT 2.0 between glycopyrrolate and glycopyrrolate related compound B
Tailing factor:
NMT 2.0 for the glycopyrrolate peak
Relative standard deviation:
NMT 6.0% for each peak
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of glycopyrrolate related compounds A, B, and C in the portion of Glycopyrrolate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of each related compound from the Sample solution |
| rS | = | = peak response of the corresponding related compound from the Standard solution |
| CS | = | = concentration of the corresponding related compound in the Standard solution (mg/mL) |
| CU | = | = concentration of Glycopyrrolate in the Sample solution (mg/mL) |
Calculate the percentage of any other individual impurity in the portion of Glycopyrrolate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rS | = | = peak response of glycopyrrolate from the Standard solution |
| CS | = | = concentration of USP Glycopyrrolate RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Glycopyrrolate in the Sample solution (mg/mL) |
Acceptance criteria:
See Table 2.
Table 2
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| 5-Nitroisophthalic acida | 0.45 | 0.15 |
| Glycopyrrolate | 1.00 | — |
| Glycopyrrolate baseb | 1.14 | 0.15 |
| Cyclopentylmandelic acidc | 2.68 | 0.15 |
| Any other individual impurity | — | 0.10 |
| Total impurities | — | 0.50 |
|
a
Glycopyrrolate related compound A.
b
Glycopyrrolate related compound B.
c
Glycopyrrolate related compound C.
|
||
Add the following:
Buffer:
2.8 g/L of monobasic sodium phosphate in water. Adjust with a sodium hydroxide solution (1 in 10) to a pH of 6.50 ± 0.05.
Mobile phase:
Methanol, acetonitrile, and Buffer (50:10:40)
System suitability solution:
40 µg/mL each of USP Glycopyrrolate Erythro Isomer RS and USP Glycopyrrolate RS in Mobile phase
Standard solution:
10 µg/mL of USP Glycopyrrolate RS in Mobile phase
Sample solution:
500 µg/mL of Glycopyrrolate in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 222 nm
Column:
4.0-mm × 25-cm; 5-µm packing L45
Column temperature:
30
Flow rate:
1 mL/min
Injection size:
10 µL
System suitability
Samples:
System suitability solution and Standard solution
Suitability requirements
Resolution:
NLT 1.2 between the erythro isomer and glycopyrrolate, System suitability solution
Tailing factor:
NMT 2.0, Standard solution
Relative standard deviation:
NMT 6.0%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of erythro isomer in the portion of Glycopyrrolate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of the erythro isomer from the Sample solution |
| rS | = | = peak response of glycopyrrolate from the Standard solution |
| CS | = | = concentration of USP Glycopyrrolate RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Glycopyrrolate in the Sample solution (mg/mL) |
Acceptance criteria:
See Table 3.
Table 3
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|
|---|---|---|---|
| Erythro isomer (R,R/S,S-glycopyrrolate)a | 0.89 | 0.4 | |
| Glycopyrrolate | 1.00 | — | |
|
a
USP Glycopyrrolate Erythro Isomer RS.
|
|||
SPECIFIC TESTS
Delete the following:
• Loss on Drying  731
731 :
Dry at 105
:
Dry at 105 for 3 h: it loses NMT 0.5% of its weight.
for 3 h: it loses NMT 0.5% of its weight.
ADDITIONAL REQUIREMENTS
Change to read:
• Packaging and Storage:
Preserve in tight containers.
Change to read:
• USP Reference Standards 5-Nitrobenzene-1,3-dicarboxylic acid.
C8H5NO6 211.13
USP Glycopyrrolate Related Compound B RS
1-Methylpyrrolidin-3-yl-2-cyclopentyl-2-hydroxy-2-phenylacetate.
C18H25NO3 303.40
1-Methylpyrrolidin-3-yl-2-cyclopentyl-2-hydroxy-2-phenylacetate.
C18H25NO3 303.40
USP Glycopyrrolate Related Compound C RS
2-Cyclopentyl-2-hydroxy-2-phenylacetic acid.
C13H16O3 220.26
2-Cyclopentyl-2-hydroxy-2-phenylacetic acid.
C13H16O3 220.26
USP Glycopyrrolate Erythro Isomer RS
(RS)-3-[(RS)-2-cyclopentyl-2-hydroxy-2-phenylacetoxy]-1,1-dimethylpyrrolidinium bromide.
C19H28BrNO3 398.33
(RS)-3-[(RS)-2-cyclopentyl-2-hydroxy-2-phenylacetoxy]-1,1-dimethylpyrrolidinium bromide.
C19H28BrNO3 398.33
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Elena Gonikberg, Ph.D.
Principal Scientific Liaison 1-301-816-8251 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3356
Pharmacopeial Forum: Volume No. 37(1)