Powdered Eleuthero
 A.
A. USP35 Thin-Layer Chromatographic Identification Test
USP35 Thin-Layer Chromatographic Identification Test  201
201
 Standard solution A:
1 mg/mL of USP Eleutheroside E RS in methanol
Standard solution A:
1 mg/mL of USP Eleutheroside E RS in methanol
 • B. HPLC:
The chromatogram of the Sample solution obtained in the test for Content of Eleutherosides B and E shows a peak at a retention time corresponding to that of eleutheroside B in the chromatogram of Standard solution B and a peak at a retention time corresponding to that of eleutheroside E in the chromatogram of Standard solution A.
• B. HPLC:
The chromatogram of the Sample solution obtained in the test for Content of Eleutherosides B and E shows a peak at a retention time corresponding to that of eleutheroside B in the chromatogram of Standard solution B and a peak at a retention time corresponding to that of eleutheroside E in the chromatogram of Standard solution A. USP35
USP35
 Standard solution A:
0.1 mg/mL of USP Eleutheroside E RS in methanol. Transfer 2.0 mL to a 5-mL volumetric flask, and dilute with Solvent to volume.
Standard solution A:
0.1 mg/mL of USP Eleutheroside E RS in methanol. Transfer 2.0 mL to a 5-mL volumetric flask, and dilute with Solvent to volume.
 System suitability
System suitability
 USP35
USP35
 2021
2021 :
:
 The total aerobic bacterial count does not exceed 105 cfu/g, the total combined molds and yeasts count does not exceed 103 cfu/g, and the bile-tolerant Gram-negative bacteria does not exceed 103 cfu/g.
The total aerobic bacterial count does not exceed 105 cfu/g, the total combined molds and yeasts count does not exceed 103 cfu/g, and the bile-tolerant Gram-negative bacteria does not exceed 103 cfu/g. USP35
USP35
 • Absence of Specified Microorganisms
• Absence of Specified Microorganisms  2022
2022 :
Meets the requirements of the tests for absence of Salmonella species and Escherichia coli
:
Meets the requirements of the tests for absence of Salmonella species and Escherichia coli USP35
USP35
 11
11
 USP Eleutheroside B RS
USP Eleutheroside B RS
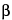 -d-Glucopyranoside, 4-(3-hydroxy-1-propenyl)-2,6-dimethoxyphenyl.
-d-Glucopyranoside, 4-(3-hydroxy-1-propenyl)-2,6-dimethoxyphenyl.
C17H24O9 372.37
 USP35
USP35
DEFINITION
Powdered Eleuthero is Eleuthero reduced to a powder or very fine powder. It contains NLT 0.08% of the sum of eleutheroside B and eleutheroside E, calculated on the dried basis.
IDENTIFICATION
Change to read:
•
Standard solution B:
1 mg/mL of USP Eleutheroside B RS in methanol
Standard solution C:
0.1 g of USP Powdered Eleuthero Extract RS in 5 mL of aqueous ethanol 50%. Sonicate for 10 min, centrifuge, and use the supernatant.
Sample solution:
Transfer about 1 g of Powdered Eleuthero to a centrifuge tube, add 5 mL of aqueous ethanol 50%, and mix well. Sonicate for 10 min. Centrifuge or filter the solution, and use the supernatant or the filtrate.
Adsorbent:
Chromatographic silica gel with an average particle size of 5 µm (HPTLC plates)
Application volume:
10 µL, as bands
Developing solvent system:
Chloroform, methanol, and water (35:15:2)
Spray reagent:
Place 18 mL of methanol in a glass flask, and cool in a water–ice–salt bath or in a freezer. To the ice-cold methanol, slowly and carefully add 2 mL of sulfuric acid, and mix well. Allow the mixture to adjust to room temperature.
Analysis
Samples:
Standard solution A, Standard solution B, Standard solution C, and Sample solution
Before the development of the chromatogram, saturate the chamber for 20 min with Developing solvent system. Record the temperature and humidity in the laboratory. If the relative humidity exceeds 50%, condition the plate to about 30% relative humidity, using a suitable device. Develop the plate over a path of 6 cm, dry, and spray with Spray reagent. Heat the plate at 100 for 5 min, and examine under visible light and UV light at 365 nm.
for 5 min, and examine under visible light and UV light at 365 nm.
Acceptance criteria:
Under visible light, the Sample solution exhibits two brown bands due to eleutheroside E and eleutheroside B at RF values of about 0.34 and 0.45, corresponding in color and RF to the bands exhibited by Standard solution A and Standard solution B, respectively. The Sample solution also exhibits two additional brown bands near the application zone, corresponding in color and RF values to the bands exhibited by Standard solution C. Other bands may be observed in the Sample solution and Standard solution C chromatograms. Under UV light, the Sample solution shows a brown band due to eleutheroside E corresponding in color and RF to the band exhibited by Standard solution A. USP35
USP35
Add the following:
COMPOSITION
Change to read:
• Content of Eleutherosides B and E
Solvent:
Methanol and water (1:1)
Solution A:
Acetonitrile and water (5:95)
Solution B:
Acetonitrile and water (60:40)
Mobile phase:
See Table 1.
Table 1
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 97 | 3 |
| 5 | 97 | 3 |
| 30 | 60 | 40 |
| 31 | 5 | 95 |
| 45 | 5 | 95 |
| 45.1 | 97 | 3 |
| 60 | 97 | 3 |
Standard solution B:
0.1 mg/mL of USP Eleutheroside B RS in methanol. Transfer 2.0 mL to a 5-mL volumetric flask, and dilute with Solvent to volume.
Standard solution C:
5.0 mg/mL of USP Powdered Eleuthero Extract RS in Solvent. Sonicate for 30 min, cool to room temperature, decant, and pass through a nylon filter of 0.45-µm or finer pore size. USP35
USP35
Sample solution:
Transfer 5.0 g of Powdered Eleuthero, accurately weighed, to a round-bottom flask equipped with a condenser. Add 50 mL of Solvent, and heat under reflux for 30 min. Filter the supernatant through cotton wool into a 100-mL volumetric flask. Transfer the cotton wool to the round-bottom flask, and repeat the extraction twice, using 22 mL of Solvent for each extraction. Filter through cotton wool into the volumetric flask, wash the residue and the cotton wool with Solvent, cool to room temperature, dilute with Solvent to volume, and mix.  Before injection, pass through a nylon filter of 0.45-µm or finer pore size, discarding the first few mL of the filtrate.
Before injection, pass through a nylon filter of 0.45-µm or finer pore size, discarding the first few mL of the filtrate. USP35
USP35
Chromatographic system
Mode:
LC
Detector:
UV 220 nm
Column:
4.0-mm × 25-cm; 5-µm packing L1
Flow rate:
1 mL/min
Injection size:
10 µL
Samples:
Standard solution B and Standard solution C
Suitability requirements
Chromatogram similarity:
The chromatogram from Standard solution C is similar to the reference chromatogram provided with the lot of USP Powdered Eleuthero Extract RS being used.
Relative standard deviation:
NMT 2.0%, determined from the eleutheroside B peak in repeated injections, Standard solution B
Analysis
 Samples:
Standard solution A, Standard solution B, Standard solution C, and Sample solution
Samples:
Standard solution A, Standard solution B, Standard solution C, and Sample solution
 USP35
USP35
Identify the eleutheroside B and eleutheroside E peaks in the Sample solution by comparison with the chromatograms of Standard solution B and Standard solution A, respectively, and measure the peak responses.
Separately calculate the percentages of eleutheroside B and eleutheroside E in the portion of Powdered Eleuthero taken:
Result = (rU/rS) × CS × (V/W) × 100
| rU | = | = peak response of the relevant analyte from the Sample solution |
| rS | = | = peak response of eleutheroside E or eleutheroside B from Standard solution A or Standard solution B, respectively |
| CS | = | = concentration of eleutheroside E or eleutheroside B in Standard solution A or Standard solution B, respectively (mg/mL) |
| V | = | = volume of the Sample solution (mL) |
| W | = | = weight of Powdered Eleuthero taken to prepare the Sample solution (mg) |
Acceptance criteria:
Add the percentages of eleutheroside B and eleutheroside E: NLT 0.08% on the dried basis.
CONTAMINANTS
• Heavy Metals, Method III  231
231 :
NMT 20 ppm
:
NMT 20 ppm
• Articles of Botanical Origin, Pesticide Residues  561
561 :
Meets the requirements
:
Meets the requirements
Change to read:
• Microbial Enumeration Tests
Add the following:
SPECIFIC TESTS
• Botanic Characteristics:
The powder is brown with a faint aromatic odor and a slightly acrid, persistent taste. Groups of secretory canals with brown contents are surrounded by parenchymatous cells containing cluster crystals of calcium oxalate. The parenchymatous cells show small starch granules, thick-walled lignified fibers, and fragments of reticulate and pitted vessels. It turns bright yellow when mounted in sodium hydroxide solution.
• Loss on Drying  731
731 :
Dry a sample at 105
:
Dry a sample at 105 to constant weight: it loses NMT 14.0% of its weight.
to constant weight: it loses NMT 14.0% of its weight.
• Articles of Botanical Origin, Total Ash  561
561 :
NMT 8.0%
:
NMT 8.0%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed, light-resistant containers.
• Labeling:
The label states the Latin binomial and, following the official name, the part of the plant from which the article was derived.
Change to read:
• USP Reference Standards C17H24O9 372.37
USP Eleutheroside E RS
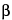 -d-Glucopyranoside, (tetrahydro-1H,3H-furo(3,4-c)furan-1,4-diyl)bis(2,6-dimethoxy-4,1-phenylene)bis-.
-d-Glucopyranoside, (tetrahydro-1H,3H-furo(3,4-c)furan-1,4-diyl)bis(2,6-dimethoxy-4,1-phenylene)bis-.
C34H46O18 742.70
C34H46O18 742.70
USP Powdered Eleuthero Extract RS
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Maged H. Sharaf, Ph.D.
Principal Scientific Liaison 1-301-816-8318 |
(DS2010) Monographs - Dietary Supplements |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1285
Pharmacopeial Forum: Volume No. 36(6) Page 1590