Gentamicin Sulfate
(jen'' ta mye' sin sul' fate).
» Gentamicin Sulfate is the sulfate salt, or a mixture of such salts, of the antibiotic substances produced by the growth of Micromonospora purpurea. It has a potency equivalent to not less than 590 µg of gentamicin per mg, calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
Labeling—
Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
pH  791
791 :
between 3.5 and 5.5, in a solution (1 in 25).
:
between 3.5 and 5.5, in a solution (1 in 25).
Loss on drying  731
731 —
Dry it in vacuum at a pressure not exceeding 5 mm of mercury at 110
—
Dry it in vacuum at a pressure not exceeding 5 mm of mercury at 110 for 3 hours: it loses not more than 18.0% of its weight.
for 3 hours: it loses not more than 18.0% of its weight.
Residue on ignition  281
281 :
not more than 1.0%.
:
not more than 1.0%.
Limit of methanol—
Internal standard solution—
Transfer 2.5 mL of n-propyl alcohol to a 500-mL volumetric flask, dilute with water to volume, and mix. This solution contains 0.50% (v/v) of n-propyl alcohol.
Standard preparation—
Transfer 1.25 mL of methanol and 1.25 mL of n-propyl alcohol to a 500-mL volumetric flask, dilute with water to volume, and mix to obtain a Standard preparation containing 0.25% (v/v) of methanol and 0.25% (v/v) of n-propyl alcohol.
Control solution—
Dissolve 0.50 g of Gentamicin Sulfate in 2.0 mL of water.
Test preparation—
Dissolve 0.50 g of Gentamicin Sulfate in 1.0 mL of Internal standard solution, add 1.0 mL of water, and mix.
Chromatographic system
(see Chromatography  621
621 )—The gas chromatograph is equipped with a flame-ionization detector and a 4-mm × 1.5-m column packed with support S3. The column temperature is maintained at a constant temperature between 120
)—The gas chromatograph is equipped with a flame-ionization detector and a 4-mm × 1.5-m column packed with support S3. The column temperature is maintained at a constant temperature between 120 and 140
and 140 , and the injection port and detector block are maintained at a constant temperature at least 50
, and the injection port and detector block are maintained at a constant temperature at least 50 higher than the column temperature. Nitrogen is used as the carrier gas at a constant flow rate of between 30 and 40 mL per minute. Chromatograph the Standard preparation, and measure the peak responses as directed under Procedure: the resolution, R, between the n-propyl alcohol peak and the methanol peak is not less than 1.0. Chromatograph the Control solution, measure the peak responses as directed under Procedure, and examine the chromatogram: if any peak is observed at a retention time corresponding to that of n-propyl alcohol, use the response of that peak to correct the n-propyl alcohol peak response in the chromatogram obtained from the Test preparation.
higher than the column temperature. Nitrogen is used as the carrier gas at a constant flow rate of between 30 and 40 mL per minute. Chromatograph the Standard preparation, and measure the peak responses as directed under Procedure: the resolution, R, between the n-propyl alcohol peak and the methanol peak is not less than 1.0. Chromatograph the Control solution, measure the peak responses as directed under Procedure, and examine the chromatogram: if any peak is observed at a retention time corresponding to that of n-propyl alcohol, use the response of that peak to correct the n-propyl alcohol peak response in the chromatogram obtained from the Test preparation.
Procedure—
Using a syringe with a polytef-tipped plunger, separately inject equal volumes (about 2 µL) of the Standard preparation and the Test preparation into the chromatograph, record the chromatograms, and measure the n-propyl alcohol and the methanol peak area responses. Calculate the percentage of methanol in the Gentamicin Sulfate taken by the formula:
1.58(P/ M)(RU / RS)
in which P is the percentage (v/v) of methanol in the Standard preparation; M is the quantity, in g, of Gentamicin Sulfate taken to prepare the Test preparation; RU is the ratio of the methanol peak area response to the n-propyl alcohol peak area response (corrected, if necessary, by subtracting the response of any peak at the locus of the n-propyl alcohol peak observed in the chromatogram of the Control solution) in the chromatogram obtained from the Test preparation; and RS is the ratio of the methanol peak area response to the n-propyl alcohol peak area response in the chromatogram obtained from the Standard preparation: not more than 1.0% of methanol is found.
Content of gentamicins—
o-Phthalaldehyde solution—
Dissolve 1.0 g of o-phthalaldehyde in 5 mL of methanol, and add 95 mL of 0.4 M boric acid, previously adjusted with 8 N potassium hydroxide to a pH of 10.4, and 2 mL of thioglycolic acid. Adjust the resulting solution with 8 N potassium hydroxide to a pH of 10.4.
Mobile phase—
Mix 700 mL of methanol, 250 mL of water, and 50 mL of glacial acetic acid. Dissolve 5 g of sodium 1-heptanesulfonate in this solution. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard preparation—
Prepare a solution of USP Gentamicin Sulfate RS in water containing about 0.65 mg per mL. Transfer 10 mL of this solution to a suitable test tube, add 5 mL of isopropyl alcohol and 4 mL of o-Phthalaldehyde solution, mix, and add isopropyl alcohol to obtain 25 mL of solution. Heat at 60 in a water bath for 15 minutes, and cool.
in a water bath for 15 minutes, and cool.
Test preparation—
Using Gentamicin Sulfate, proceed as directed for Standard preparation.
Chromatographic system
(see Chromatography  621
621 )—The liquid chromatograph is equipped with a 330-nm detector and a 5-mm × 10-cm column that contains 5-µm packing L1. The flow rate is about 1.5 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed under Procedure: the resolution, R, between any two peaks is not less than 1.25, the capacity factor determined from the gentamicin C1 peak is between 2 and 7, the column efficiency determined from the gentamicin C2 peak is not less than 1200 theoretical plates, and the relative standard deviation for replicate injections is not more than 2.0%.
)—The liquid chromatograph is equipped with a 330-nm detector and a 5-mm × 10-cm column that contains 5-µm packing L1. The flow rate is about 1.5 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed under Procedure: the resolution, R, between any two peaks is not less than 1.25, the capacity factor determined from the gentamicin C1 peak is between 2 and 7, the column efficiency determined from the gentamicin C2 peak is not less than 1200 theoretical plates, and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
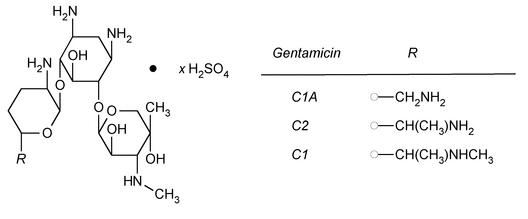
Separately inject equal volumes (about 20 µL) of the Standard preparation and the Test preparation into the chromatograph, record the chromatograms, and measure the area responses for the major peaks. The elution order is gentamicin C1, gentamicin C1a, gentamicin C2a, and gentamicin C2. Calculate the percentage contents of gentamicin C1, gentamicin C1a, gentamicin C2a, and gentamicin C2 in the portion of Gentamicin Sulfate taken by the formula:
100rf / rs
in which rf is the peak area response corresponding to the particular gentamicin; and rs is the sum of the area responses of all four peaks: the content of gentamicin C1 is between 25% and 50%, the content of gentamicin C1a is between 10% and 35%, and the sum of the contents of gentamicin C2a and gentamicin C2 is between 25% and 55%.
Other requirements—
Where the label states that Gentamicin Sulfate is sterile, it meets the requirements for Sterility Tests  71
71 and for Bacterial endotoxins in Gentamicin Injection. Where the label states that Gentamicin Sulfate must be subjected to further processing during the preparation of injectable dosage forms, it meets the requirements for Bacterial endotoxins in Gentamicin Injection.
and for Bacterial endotoxins in Gentamicin Injection. Where the label states that Gentamicin Sulfate must be subjected to further processing during the preparation of injectable dosage forms, it meets the requirements for Bacterial endotoxins in Gentamicin Injection.
Assay—
Proceed with Gentamicin Sulfate as directed under Antibiotics—Microbial Assays  81
81 .
.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ahalya Wise, M.S.
Senior Scientific Liaison 1-301-816-8161 |
(SM12010) Monographs - Small Molecules 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3325