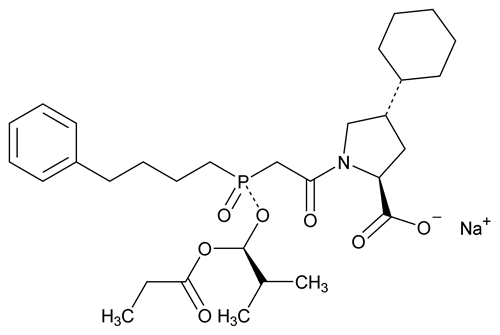
Fosinopril Sodium
C30H45NNaO7P 585.64
l-Proline, 4-cyclohexyl-1-[[[2-methyl-1-(1-oxopropoxy)propoxy](4-phenylbutyl)phosphinyl]acetyl]-, sodium salt, [1[S*(R*)],2 ,4
,4 ]-;
]-;
(4S)-4-Cyclohexyl-1-[(R)-[(S)-1-hydroxy-2-methylpropoxy](4-phenylbutyl)phosphinyl]acetyl-l-proline propionate (ester), sodium salt

 [88889-14-9].
[88889-14-9].
(fos in' oh pril soe' dee um).
C30H45NNaO7P 585.64
l-Proline, 4-cyclohexyl-1-[[[2-methyl-1-(1-oxopropoxy)propoxy](4-phenylbutyl)phosphinyl]acetyl]-, sodium salt, [1[S*(R*)],2
(4S)-4-Cyclohexyl-1-[(R)-[(S)-1-hydroxy-2-methylpropoxy](4-phenylbutyl)phosphinyl]acetyl-l-proline propionate (ester), sodium salt
DEFINITION
Fosinopril Sodium contains NLT 97.5% and NMT 102.0% of C30H45NNaO7P, calculated on the anhydrous basis.
IDENTIFICATION
ASSAY
• Procedure
Mobile phase:
Acetonitrile, phosphoric acid, and water (2000:1:10)
System suitability solution:
0.1 mg/mL of USP Fosinopril Sodium RS and 0.01 mg/mL of USP Fosinopril Related Compound B RS in Mobile phase
Standard solution:
0.10 mg/mL of USP Fosinopril Sodium RS in Mobile phase
Sample solution:
0.10 mg/mL of Fosinopril Sodium in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 214 nm
Column:
3.9-mm × 15-cm; packing L3
Temperature:
33
Flow rate:
1.2 mL/min
Injection size:
20 µL
System suitability
Sample:
System suitability solution
Suitability requirements
Resolution:
NLT 2.0 between fosinopril related compound B and fosinopril sodium
Relative standard deviation:
NMT 2.0% for Fosinopril peak
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C30H45NNaO7P in the portion of Fosinopril Sodium taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Fosinopril Sodium RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of Fosinopril Sodium in the Sample solution (mg/mL) |
Acceptance criteria:
97.5%–102.0% on the anhydrous basis
IMPURITIES
Inorganic Impurities
• Heavy Metals, Method II  231
231 :
20 ppm
:
20 ppm
Organic Impurities
• Procedure 1
Mobile phase, Sample solution and Chromatographic system:
Proceed as directed in the Assay. [Note—Record the chromatograms for four times the retention time of the fosinopril sodium peak. ]
System suitability solution:
0.1 mg/mL of USP Fosinopril Sodium RS and 0.01 mg/mL each of USP Fosinopril Related Compound A RS and USP Fosinopril Related Compound B RS in Mobile phase
Analysis
Sample:
Sample solution
Calculate the percentage of each individual related compound:
Result = (rU/rT) × 100
| rU | = | = individual peak response, other than the fosinopril sodium peak |
| rT | = | = sum of all the peak responses |
[Note—If present, two more diastereomers may not be resolved from fosinopril related compound B by this method. These peaks, appearing at a relative retention time of 0.7, should be integrated together to determine conformance with the limit in Impurity Table 1. ]
Acceptance criteria
Individual impurities:
See Impurity Table 1.
Impurity Table 1
| Name | Relative Retention Time |
Procedure | Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Fosinopril Related Compound Aa | 2.0 | 1 | 0.75 |
| Fosinopril Related Compound Bb | 0.7 | 1 | 1.0 |
| Fosinopril Related Compound Cc | 1.2 | 2 | 0.3 |
| Fosinopril Related Compound Dd | 1.3 | 2 | 0.3 |
| Fosinopril Related Compound Ee | 0.8 | 3 | 0.3 |
| Fosinopril Related Compound Ff | 0.9 | 3 | 0.3 |
| Impurity 1g | 0.53 | 1 | 0.3 |
| Impurity 2h | 0.67 | 1 | 0.2 |
| Impurity 3 (if present)i | 0.37 | 1 | 0.15 |
|
a
(4S)-4-Cyclohexyl-1-[(4-phenylbutyl)phosphinyl]acetyl-l-proline.
b
(4S)-4-Cyclohexyl-1-[(R)-[(S)-1-hydroxy-2-methylpropoxy](4-phenylbutyl)phosphinyl]acetyl-d-proline propionate (ester).
c
Mixture of (4S)-4-cyclohexyl-1-[(S)-[(S)-1-hydroxy-2-methylpropoxy](4-phenylbutyl)phosphinyl]acetyl-l-proline propionate (ester), sodium salt and (4S)-4-cyclohexyl-1-[[(R)-[(R)-1-hydroxy-2-methylpropoxy](4-phenylbutyl)phosphinyl]acetyl-l-proline propionate (ester), sodium salt.
d
(4R)-4-Cyclohexyl-1-[(R)-[(S)-1-hydroxy-2-methylpropoxy](4-phenylbutyl)phosphinyl]acetyl-l-proline propionate (ester), sodium salt.
e
(4S)-4-Phenyl-1-[(R)-[(S)-1-hydroxy-2-methylpropoxy](4-phenylbutyl)phosphinyl]acetyl-l-proline propionate (ester), sodium salt.
f
(4S)-4-Cyclohexyl-1-[(R)-[(S)-1-hydroxy-propoxy](4-phenylbutyl)phosphinyl]acetyl-l-proline propionate (ester),sodium salt.
g
(2S,4S)-4-Cyclohexyl-1-pivaloylpyrrolidine-2-carboxylic acid.
h
2-((RS)-((SR)-2-Methyl-1-(propionyloxy)propoxy)(4-phenylbutyl)phosphinyl)acetic acid.
i
(S)-4-cyclohexyl-1-(3-oxopentanoyl)-l-proline.
|
|||
• Procedure 2
Sample solution:
Proceed as directed in the Assay.
Mobile phase:
Acetonitrile, phosphoric acid, and water (4000:2:15)
System suitability solution:
0.1 mg/mL of USP Fosinopril Sodium RS and 0.01 mg/mL each of USP Fosinopril Related Compound C RS and USP Fosinopril Related Compound D RS in the Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 214 nm
Column:
4.6-mm × 25-cm; packing L12
Temperature:
45
Flow rate:
0.9 mL/min
Injection size:
20 µL
Run time:
Twice the retention time of fosinopril sodium
System suitability
Sample:
System suitability solution
Suitability requirements
Resolution:
NLT 1.5 between fosinopril sodium and fosinopril related compound C
Analysis
Sample:
Sample solution
Calculate the percentages of fosinopril related compound C and fosinopril related compound D only:
Result = (rU/rT) × 100
| rU | = | = peak response of fosinopril related compound C or fosinopril related compound D |
| rT | = | = sum of all the peak responses |
• Procedure 3
Solution A:
1-in-500 solution of phosphoric acid
Mobile phase:
Acetonitrile and Solution A (14:11)
System suitability solution:
0.01 mg/mL each of USP Fosinopril Sodium RS, USP Fosinopril Related Compound E RS, and USP Fosinopril Related Compound F RS in Mobile phase
Sample solution:
0.2 mg/mL of Fosinopril Sodium in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 205 nm
Column:
4.6-mm × 25-cm; packing L11
Temperature:
45
Flow rate:
1 mL/min
Injection size:
20 µL
Run time:
Four times the retention time of fosinopril sodium
System suitability
Sample:
System suitability solution
Suitability requirements
Resolution:
NLT 1.5 between fosinopril related compound F and fosinopril sodium; NLT 1.5 between fosinopril related compound E and fosinopril related compound F
Analysis
Sample:
Sample solution
Measure the peak responses, carrying out the chromatography to four times the retention time of the fosinopril sodium peak.
Calculate the percentages of fosinopril related compound E and fosinopril related compound F only:
Result = (rU/rT) × 100
| rU | = | = peak response of fosinopril related compound E or fosinopril related compound F |
| rT | = | = sum of all the peak responses |
Acceptance criteria
Individual impurities:
See Impurity Table 1. In addition to not exceeding the limits for impurities in Impurity Table 1, NMT 0.1% of any other individual impurity is found (calculated as directed in Procedure 1).
Total impurities:
NMT 1.5% for the total of Procedure 1, Procedure 2, and Procedure 3
SPECIFIC TESTS
• Water Determination, Method I  921
921 :
NMT 0.2%
:
NMT 0.2%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers, and store at controlled room temperature.
• USP Reference Standards  11
11
USP Fosinopril Related Compound A RS 
(4S)-4-Cyclohexyl-[(4-phenylbutyl)phosphinyl]acetyl-l-proline.
C23H34NO5P 435.49

(4S)-4-Cyclohexyl-[(4-phenylbutyl)phosphinyl]acetyl-l-proline.
C23H34NO5P 435.49
USP Fosinopril Related Compound B RS 
(4S)-4-Cyclohexyl-1-[(R)-[(S)-1-hydroxy-2-methylpropoxy](4-phenylbutyl)phosphinyl]acetyl-d-proline propionate (ester), hemibarium salt, sesquihydrate.
C30H45NO7P·½Ba·1½H2O 658.34

(4S)-4-Cyclohexyl-1-[(R)-[(S)-1-hydroxy-2-methylpropoxy](4-phenylbutyl)phosphinyl]acetyl-d-proline propionate (ester), hemibarium salt, sesquihydrate.
C30H45NO7P·½Ba·1½H2O 658.34
USP Fosinopril Related Compound C RS 
(4S)-4-Cyclohexyl-1-[(RS)-[(RS)-1-hydroxy-2-methylpropoxy](4-phenylbutyl)phosphinyl]acetyl-l-proline propionate (ester), sodium salt.
C30H45NNaO7P 585.64

(4S)-4-Cyclohexyl-1-[(RS)-[(RS)-1-hydroxy-2-methylpropoxy](4-phenylbutyl)phosphinyl]acetyl-l-proline propionate (ester), sodium salt.
C30H45NNaO7P 585.64
USP Fosinopril Related Compound D RS 
(4R)-4-Cyclohexyl-1-[(R)-[(S)-1-hydroxy-2-methylpropoxy](4-phenylbutyl)phosphinyl]acetyl-l-proline propionate (ester), sodium salt.
C30H45NNaO7P 585.64

(4R)-4-Cyclohexyl-1-[(R)-[(S)-1-hydroxy-2-methylpropoxy](4-phenylbutyl)phosphinyl]acetyl-l-proline propionate (ester), sodium salt.
C30H45NNaO7P 585.64
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Sujatha Ramakrishna, Ph.D.
Senior Scientific Liaison 1-301-816-8349 |
(SM22010) Monographs - Small Molecules 2 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3281
Pharmacopeial Forum: Volume No. 35(5) Page 1144