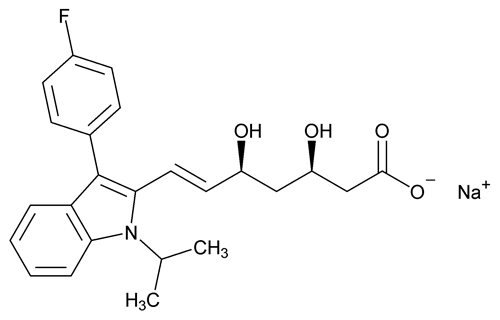
Fluvastatin Sodium
C24H25FNNaO4 433.45
6-Heptenoic acid, 7-[3-(4-fluorophenyl)-1-(1-methylethyl)-1H-indol-2-yl]-3,5-dihydroxy-, monosodium salt, [R*,S*-(E)]-(±)-;
Sodium (±)-(3R*,5S*,6E)-7-[3-(p-fluorophenyl)-1-isopropylindol-2-yl]-3,5-dihydroxy-6-heptenoate

 [93957-55-2].
[93957-55-2].
(floo'' va stat' in soe' dee um).
C24H25FNNaO4 433.45
6-Heptenoic acid, 7-[3-(4-fluorophenyl)-1-(1-methylethyl)-1H-indol-2-yl]-3,5-dihydroxy-, monosodium salt, [R*,S*-(E)]-(±)-;
Sodium (±)-(3R*,5S*,6E)-7-[3-(p-fluorophenyl)-1-isopropylindol-2-yl]-3,5-dihydroxy-6-heptenoate
DEFINITION
Fluvastatin Sodium contains NLT 98.0% and NMT 102.0% of C24H25FNNaO4, calculated on the anhydrous basis.
IDENTIFICATION
• A. Infrared Absorption  197K
197K
[Note—If a difference appears in the IR spectra of the analyte and the Standard, dissolve equal portions of the sample specimen and the USP Reference Standard in equal volumes of methanol. Evaporate the solutions to dryness on a steam bath, protecting the solutions from light, and dry at 105 for 30 min. Repeat the test on the residues. ]
for 30 min. Repeat the test on the residues. ]
• B. Identification Tests—General, Sodium  191
191 :
Meets the requirements for the pyroantimonate precipitation test
:
Meets the requirements for the pyroantimonate precipitation test
ASSAY
• Procedure
Solution A:
Add 20 mL of 25% aqueous tetramethylammonium hydroxide solution to 880 mL of water. Adjust with about 2.3 mL of phosphoric acid to a pH of 7.2 ± 0.2. Add 100 mL of a mixture of methanol and acetonitrile (3:2).
Solution B:
Add 20 mL of 25% aqueous tetramethylammonium hydroxide solution and 80 mL of water to 900 mL of a mixture of methanol and acetonitrile (3:2). Adjust with about 2.3 mL of phosphoric acid to a pH of 7.2 ± 0.2.
Mobile phase:
See the gradient table.
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 60 | 40 |
| 6 | 60 | 40 |
| 20 | 18 | 82 |
| 20.1 | 60 | 40 |
| 25.1 | 60 | 40 |
System suitability solution:
0.5 mg/mL of fluvastatin sodium from USP Fluvastatin for System Suitability RS, dissolved first in Solution B, using 40% of the final volume, then diluted with Solution A to volume
Standard solution:
0.5 mg/mL of USP Fluvastatin Sodium RS, dissolved first in Solution B, using 40% of the final volume, then diluted with Solution A to volume
Sample solution:
0.5 mg/mL of Fluvastatin Sodium, dissolved first in Solution B, using 40% of the final volume, then diluted with Solution A to volume
Chromatographic system
Mode:
LC
Detector:
UV 305 nm
Column:
4.6-mm × 5-cm; 5-µm packing L1
Column temperature:
35
Flow rate:
3 mL/min
Injection size:
20 µL
[Note—Adjust the start time of the gradient step and the equilibration time for each instrument. ]
System suitability
Samples:
System suitability solution and Standard solution
[Note—The relative retention times for fluvastatin and fluvastatin anti-isomer are about 1.0 and 1.2, respectively. ]
Suitability requirements
Resolution:
NLT 1.6 between fluvastatin anti-isomer and fluvastatin, System suitability solution
Column efficiency:
NLT 700 theoretical plates for the fluvastatin peak, System suitability solution
Tailing factor:
NMT 3.0 for the fluvastatin peak, System suitability solution
Relative standard deviation:
NMT 1.0%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C24H25FNNaO4 in the portion of Fluvastatin Sodium taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Fluvastatin Sodium RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Fluvastatin Sodium in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% on the anhydrous basis
IMPURITIES
Inorganic Impurities
• Heavy Metals, Method II  231
231 :
20 ppm
:
20 ppm
Organic Impurities
• Procedure
[Note—Protect all solutions from light, and use amber autosampler vials and low-actinic glassware. ]
Solution A, Solution B, Mobile phase, Standard solution, and Sample solution:
Proceed as directed in the Assay.
System suitability solution A:
Prepare as directed for the System suitability solution in the Assay.
System suitability solution B:
0.1 mg/mL of USP Fluvastatin Related Compound B RS in a mixture of methanol and acetonitrile (3:2). Transfer about 0.5 mL of this solution to a 10-mL volumetric flask, and dilute with System suitability solution A to volume. [Note—System suitability solution B is stable for up to 6 months if stored in a refrigerator. ]
Chromatographic system:
Proceed as directed in the Assay, except to use a liquid chromatograph equipped with either a programmable variable-wavelength detector or two separate detectors capable of monitoring at 305 and 365 nm.
System suitability
Samples:
Standard solution and System suitability solution B
[Note—Record the peak responses at 305 nm as directed for Analysis. Identify the peaks corresponding to fluvastatin, fluvastatin anti-isomer, and fluvastatin t-butyl ester. ]
Suitability requirements
Resolution:
NLT 1.6 between fluvastatin anti-isomer and fluvastatin, System suitability solution B
Column efficiency:
NLT 700 theoretical plates for the fluvastatin peak, System suitability solution B
Tailing factor:
NMT 3.0 for the fluvastatin peak, System suitability solution B
Relative standard deviation:
NMT 1.0% at 305 nm, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Record the chromatograms at 305 and 365 nm, identify the impurities listed in Impurity Table 1, and measure the peak responses. [Note—3-Hydroxy-5-keto fluvastatin is monitored using a wavelength of 365 nm, and all other compounds are monitored at 305 nm. ]
Calculate the percentage of each impurity, except for 3-hydroxy-5-keto fluvastatin, in the portion of Fluvastatin Sodium taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
| rU | = | = peak response at 305 nm of each impurity from the Sample solution |
| rS | = | = peak response at 305 nm of fluvastatin from the Standard solution |
| CS | = | = concentration of USP Fluvastatin Sodium RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Fluvastatin Sodium in the Sample solution (mg/mL) |
| F | = | = relative response factor (see Impurity Table 1) |
Calculate the percentage of 3-hydroxy-5-keto fluvastatin in the portion of Fluvastatin Sodium taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
| rU | = | = peak response at 365 nm of 3-hydroxy-5-keto fluvastatin from the Sample solution |
| rS | = | = peak response at 365 nm of fluvastatin from the Standard solution |
| CS | = | = concentration of USP Fluvastatin Sodium RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Fluvastatin Sodium in the Sample solution (mg/mL) |
| F | = | = relative response factor (see Impurity Table 1) |
Acceptance criteria
Individual Impurities:
See Impurity Table 1.
Total impurities:
NMT 1.0%
Impurity Table 1
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Fluvastatin N-ethyl analoga | 0.7 | 1.2 | 0.1 |
| Fluvastatin anti-isomerb | 1.2 | 1.0 | 0.8 |
| 3-Hydroxy-5-keto fluvastatinc | 1.5 | 27.0d | 0.1 |
| Fluvastatin hydroxydienee |
2.0 | 0.92 | 0.1 |
| Fluvastatin short-chain aldehydef | 3.0 | 1.4 | 0.1 |
| Fluvastatin t-butyl ester (fluvastatin related compound B)g | 3.4 | 0.94 | 0.2 |
| Any other individual impurity |
— | 1.0 | 0.1 |
|
a
[R*,S*-E]-(±)-7-[3-(4-Fluorophenyl)-1-ethyl-1H-indol-2-yl]-3,5-dihydroxy-6-heptenoic acid monosodium salt.
b
[R*,R*-E]-(±)-7-[3-(4-Fluorophenyl)-1-(methylethyl)-1H-indol-2-yl]-3,5-dihydroxy-6-heptenoic acid monosodium salt.
c
E-(±)-7-[3-(4-Fluorophenyl)-1-(methylethyl)-1H-indol-2-yl]-3-hydroxy-5-oxo-6-heptenoic acid monosodium salt.
d
At 365 nm.
e
[E,E]-(±)-7-[3-(4-Fluorophenyl)-1-(methylethyl)-1H-indol-2-yl]-3-hydroxy-4,6-heptadienoic acid monosodium salt.
f
3-(4-Fluorophenyl)-1-(methylethyl)-1H-indole-2-carboxaldehyde.
g
[R*,S*-E]-(±)-7-[3-(4-Fluorophenyl)-1-methylethyl-1H-indol-2-yl]-3,5-dihydroxy-6-heptenoic acid 1,1-dimethylethyl ester.
|
|||
SPECIFIC TESTS
• pH  791
791 :
8.0–10.0, in a 10-mg/mL solution. Perform the test immediately after preparation.
:
8.0–10.0, in a 10-mg/mL solution. Perform the test immediately after preparation.
• Water Determination, Method I  921
921 :
NMT 4.0%; if labeled as a hydrated form, NMT 12.0%. [Note—For this monograph, the term “hydrated form” refers to several known forms of fluvastatin sodium that are not stoichiometric hydrates. ]
:
NMT 4.0%; if labeled as a hydrated form, NMT 12.0%. [Note—For this monograph, the term “hydrated form” refers to several known forms of fluvastatin sodium that are not stoichiometric hydrates. ]
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers, protected from moisture. Store at a temperature not exceeding 40 .
.
• Labeling:
Where it is a hydrated form, the label so indicates.
• USP Reference Standards  11
11
USP Fluvastatin Related Compound B RS
Fluvastatin t-butyl ester.
Fluvastatin t-butyl ester.
USP Fluvastatin for System Suitability RS
Fluvastatin sodium, containing 1% to 2% of the fluvastatin sodium anti-isomer.
Fluvastatin sodium, containing 1% to 2% of the fluvastatin sodium anti-isomer.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Elena Gonikberg, Ph.D.
Principal Scientific Liaison 1-301-816-8251 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3268
Pharmacopeial Forum: Volume No. 36(4) Page 921