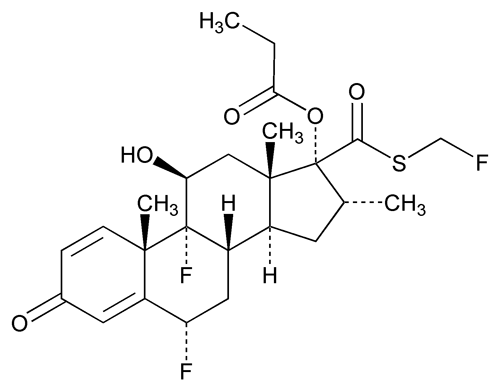
Fluticasone Propionate
C25H31F3O5S 500.57
Androsta-1,4-diene-17-carbothioic acid, 6,9-difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)-, (6 ,11
,11 ,16
,16 ,17
,17 )-S-(fluoromethyl) ester;
)-S-(fluoromethyl) ester;
S-Fluoromethyl 6 ,9
,9 -difluoro-11
-difluoro-11 -hydroxy-16
-hydroxy-16 -methyl-3-oxo-17
-methyl-3-oxo-17 -propionyloxyandrosta-1,4-diene-17
-propionyloxyandrosta-1,4-diene-17 -carbothioate
-carbothioate


 [80474-14-2].
[80474-14-2].
(floo tik' a sone proe' pee oh nate).
C25H31F3O5S 500.57
Androsta-1,4-diene-17-carbothioic acid, 6,9-difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)-, (6
S-Fluoromethyl 6
DEFINITION
Fluticasone Propionate contains NLT 98.0% and NMT 101.0% of C25H31F3O5S, calculated on the anhydrous, solvent-free basis.
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
Buffer:
1.15 g/L of monobasic ammonium phosphate, adjusted with phosphoric acid to a pH of 3.5 ± 0.05
Mobile phase:
Methanol, acetonitrile, and Buffer (50:15:35)
System suitability solution:
0.05 mg/mL of USP Fluticasone Propionate Resolution Mixture RS in Mobile phase
Standard solution:
0.04 mg/mL of USP Fluticasone Propionate RS in Mobile phase
Sample solution:
0.04 mg/mL of Fluticasone Propionate in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 239 nm
Column:
4.6-mm × 25-cm; 5-µm packing L1
Column temperature:
40
Flow rate:
1.5 mL/min
Injection size:
20 µL
System suitability
Samples:
System suitability solution and Standard solution
[Note—The relative retention times for fluticasone propionate and fluticasone propionate related compound D are about 1.0 and 1.10, respectively. ]
Suitability requirements
Resolution:
NLT 1.5 between fluticasone propionate and fluticasone propionate related compound D, System suitability solution
Relative standard deviation:
NMT 2%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of fluticasone propionate (C25H31F3O5S) in the portion of Fluticasone Propionate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of fluticasone propionate from the Sample solution |
| rS | = | = peak response of fluticasone propionate from the Standard solution |
| CS | = | = concentration of USP Fluticasone Propionate RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Fluticasone Propionate in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–101.0% on the anhydrous, solvent-free basis
IMPURITIES
• Organic Impurities
Solution A:
0.5 mL of phosphoric acid in 1000 mL of acetonitrile
Solution B:
0.5 mL of phosphoric acid in 1000 mL of methanol
Solution C:
0.5 mL of phosphoric acid in 1000 mL of water
Mobile phase:
See Table 1.
Table 1
| Time (min) |
Solution A (%) |
Solution B (%) |
Solution C (%) |
|---|---|---|---|
| 0 | 42 | 3 | 55 |
| 40 | 53 | 3 | 44 |
| 60 | 87 | 3 | 10 |
| 70 | 87 | 3 | 10 |
| 75 | 42 | 3 | 55 |
System suitability solution:
Dissolve 2.0 mg of USP Fluticasone Propionate System Suitability Mixture RS in 5 mL of Solution A using sonication. Add 5 mL of Solution C.
Sample solution:
2.0 mg/mL prepared as follows: Dissolve 2.0 mg of Fluticasone Propionate in 5 mL of Solution A using sonication. Add 5 mL of Solution C.
Chromatographic system
Mode:
LC
Detector:
UV 239 nm
Column:
4.6-mm × 25-cm; 5-µm packing L1
Column temperature:
40
Flow rate:
1 mL/min
Injection size:
50 µL
System suitability
Sample:
System suitability solution
Suitability requirements
Resolution:
NLT 0.6 between fluticasone propionate related compound B and fluticasone propionate related compound C; NLT 1.5 between fluticasone propionate related compound D and fluticasone propionate
Analysis
Sample:
Sample solution
Calculate the percentage of each impurity in the portion of Fluticasone Propionate taken:
Result = (rU/rT) × 100
| rU | = | = peak response for each impurity |
| rT | = | = sum of the responses of all the peaks |
Acceptance criteria:
See Table 2.
Table 2
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Fluticasone propionate related compound A | 0.5 | 0.2 |
| Fluticasone propionate related compound B | 0.75 | 0.1 |
| Fluticasone propionate related compound C | 0.8 | 0.1 |
| Fluticasone propionate related compound D | 0.95 | 0.3 |
| Fluticasone propionate | 1.0 | — |
| Fluticasone propionate related compound E | 1.3 | 0.3 |
| Any individual unspecified impurity | — | 0.1 |
| Total impurities* | — | 1.0 |
|
*
Include all impurity peaks greater than or equal to 0.05%.
|
||
• Limit of Acetone
Internal standard solution:
0.05% (v/v) solution of tetrahydrofuran in dimethylformamide
Standard solution:
0.05% (v/v) of acetone in Internal standard solution
Sample solution:
50 mg/mL of Fluticasone Propionate in the Internal standard solution
Chromatographic system
Mode:
GC
Detector:
Flame ionization
Column:
0.53-mm × 25-m; 2-µm film of phase G16
Carrier gas:
Nitrogen or helium
Temperature
Detector:
250
Splitless injector:
150
Column:
See Table 3.
Table 3
| Initial Temperature ( |
Temperature Ramp ( |
Final Temperature ( |
Hold Time at Final Temperature (min) |
|---|---|---|---|
| 60 | 0 | 60 | 3.5 |
| 60 | 30 | 180 | 3.0 |
Flow rate:
5.5 mL/min
Injection size:
0.1 µL
System suitability
Sample:
Standard solution
Suitability requirements
Relative standard deviation:
NMT 5.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of acetone (w/w) in the portion of Fluticasone Propionate taken:
Result = (RU/RS) × D × (CS/CU)
| RU | = | = peak response ratio of acetone to tetrahydrofuran from the Sample solution |
| RS | = | = peak response ratio of acetone to tetrahydrofuran from the Standard solution |
| D | = | = density of acetone at 20 |
| CS | = | = concentration of acetone in the Standard solution (%v/v) |
| CU | = | = concentration of Fluticasone Propionate in the Sample solution (g/mL) |
Acceptance criteria:
NMT 1.0% (w/w)
SPECIFIC TESTS
• Optical Rotation, Specific Rotation  781S
781S :
+32
:
+32 to +36
to +36 at 20
at 20 , calculated on the anhydrous, solvent-free basis
, calculated on the anhydrous, solvent-free basis
Sample solution:
0.5% (w/v) of Fluticasone Propionate in dichloromethane (0.5 g in 100 mL)
• Water Determination, Method I  921
921 :
NMT 0.2% (w/w)
:
NMT 0.2% (w/w)
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers, and store at a temperature not exceeding 30 .
.
• Labeling:
Fluticasone Propionate in the form of microcrystals is so labeled.
• USP Reference Standards  11
11
USP Fluticasone Propionate Resolution Mixture RS
USP Fluticasone Propionate System Suitability Mixture RS
It is a mixture of USP Fluticasone Propionate RS and fluticasone propionate related compounds B, C, and D.
and fluticasone propionate related compounds B, C, and D.
Fluticasone propionate related compound A: 6 ,9
,9 -Difluoro-11
-Difluoro-11 -hydroxy-16
-hydroxy-16 -methyl-3-oxo-17
-methyl-3-oxo-17 -propionyloxyandrosta-1,4-diene-17
-propionyloxyandrosta-1,4-diene-17 -carbonylsulfenic acid.
-carbonylsulfenic acid.
Fluticasone propionate related compound B: 6 ,9
,9 -Difluoro-11
-Difluoro-11 -hydroxy-16
-hydroxy-16 -methyl-2¢,3,4¢-trioxo-17
-methyl-2¢,3,4¢-trioxo-17 -spiro(androsta-1,4-diene-17,5¢-(1,3)oxathiolane).
-spiro(androsta-1,4-diene-17,5¢-(1,3)oxathiolane).
Fluticasone propionate related compound C: S-Fluoromethyl 17 -acetyloxy-6
-acetyloxy-6 ,9
,9 -difluoro-11
-difluoro-11 -hydroxy-16
-hydroxy-16 -methyl-3-oxo-androsta-1,4-diene-17
-methyl-3-oxo-androsta-1,4-diene-17 -carbothioate.
-carbothioate.
Fluticasone propionate related compound D: S-Methyl 6 ,9
,9 -difluoro-11
-difluoro-11 -hydroxy-16
-hydroxy-16 -methyl-3-oxo-17
-methyl-3-oxo-17 -propionyloxy-androsta-1,4-diene-17
-propionyloxy-androsta-1,4-diene-17 -carbothioate.
-carbothioate.
Fluticasone propionate related compound E: 6 ,9
,9 -Difluoro-11
-Difluoro-11 ,17
,17 -dihydroxy-16
-dihydroxy-16 -methyl-3-oxo-androsta-1,4-diene-17
-methyl-3-oxo-androsta-1,4-diene-17 -carboxylic acid 6
-carboxylic acid 6 ,9
,9 -difluoro-17
-difluoro-17 -(fluoromethylthio) carbonyl-11
-(fluoromethylthio) carbonyl-11 -hydroxy-16
-hydroxy-16 -methyl-3-oxo-androsta-1,4-dien-17
-methyl-3-oxo-androsta-1,4-dien-17 -yl ester.
-yl ester.
It is a mixture of USP Fluticasone Propionate RS
 and fluticasone propionate related compounds B, C, and D.
and fluticasone propionate related compounds B, C, and D.
Fluticasone propionate related compound A: 6
Fluticasone propionate related compound B: 6
Fluticasone propionate related compound C: S-Fluoromethyl 17
Fluticasone propionate related compound D: S-Methyl 6
Fluticasone propionate related compound E: 6
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ravi Ravichandran, Ph.D.
Principal Scientific Liaison 1-301-816-8330 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3261
Pharmacopeial Forum: Volume No. 32(2) Page 337