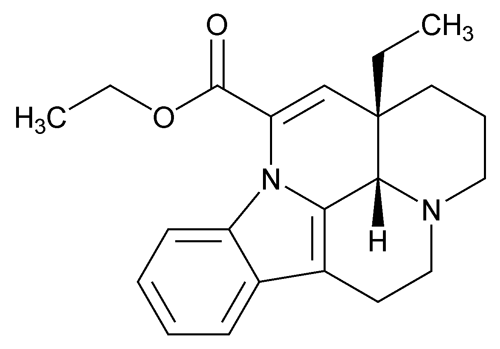
Vinpocetine
C22H26N2O2 350.45
Eburnamenine-14-carboxylic acid, ethyl ester, (3 ,16
,16 )-;
)-;
Ethyl apovincamin-22-oate

 [42971-09-5].
[42971-09-5].
(vin poe' se teen).
C22H26N2O2 350.45
Eburnamenine-14-carboxylic acid, ethyl ester, (3
Ethyl apovincamin-22-oate
DEFINITION
Vinpocetine contains NLT 98.5% and NMT 101.5% of C22H26N2O2, calculated on the dried basis.
IDENTIFICATION
• B.
The retention time of the main peak of the Sample solution corresponds to that of the principal peak for the Stock standard solution, as obtained in the test for Organic Impurities.
ASSAY
• Titrimetry
(See Titrimetry  541
541 .)
.)
Sample:
300 mg of Vinpocetine in 50 mL of a mixture of acetic anhydride and acetic acid (1:1)
Titrimetric system
Mode:
Direct titration
Titrant:
0.1 N perchloric acid VS
Endpoint detection:
Potentiometric
Analysis
Perform a blank determination, and make any necessary correction. Calculate the percentage of C22H26N2O2 in the Sample taken:
Result = [(V  B) × N × F × 100]/W
B) × N × F × 100]/W
| V | = | = volume of titrant consumed by the Sample (mL) |
| B | = | = volume of titrant consumed by the blank (mL) |
| N | = | = normality of the titrant (mEq/mL) |
| F | = | = equivalency factor, 350.5 mg/mEq |
| W | = | = Sample weight (mg) |
Acceptance criteria:
98.5%–101.5% on the dried basis
IMPURITIES
Organic Impurities
• Procedure
Calculate the percentage of any unspecified individual impurity, as vinpocetine, in the portion of Vinpocetine taken:
Ammonium acetate solution:
15.4 g/L ammonium acetate in water
Mobile phase:
Acetonitrile and Ammonium acetate solution (55:45)
Sample solution:
1.00 mg/mL of Vinpocetine in Mobile phase
Stock standard solution:
0.02 mg/mL of USP Vinpocetine RS in Mobile phase
Standard solution 1:
0.12 mg/mL of USP Vinpocetine Related Compound A RS and 0.10 mg/mL each of USP Vinpocetine Related Compound B RS, USP Vinpocetine Related Compound C RS, and USP Vinpocetine Related Compound D RS in Mobile phase
Standard solution 2:
Dilute 1.0 mL of Stock standard solution and 1.0 mL of Standard solution 1 with Mobile phase to 20.0 mL.
Chromatographic system
Mode:
LC
Detector:
280 nm
Column:
4.6-mm × 25-cm; 5-µm packing L1
Flow rate:
1.0 mL/min
Chromatograph the Stock standard solution and Standard solution 2, and identify the vinpocetine peak and peaks due to the related compounds listed in Impurity Table 1.
Injection size:
15 µL (duplicate equal volumes)
System suitability
Sample:
Standard solution 2
Suitability requirements
Resolution:
NLT 2.0 between vinpocetine related compound B and vinpocetine related compound D
Analysis
Samples:
Sample solution and Standard solution 2
Record the chromatograms for up to a minimum of three times the retention time of vinpocetine. Disregard any peak with an area less than 0.5 times the area of the peak due to vinpocetine in Standard solution 2. Calculate the percentage of vinpocetine related compounds A, B, C, and D in the portion of Vinpocetine taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of each of the known impurities from the Sample solution |
| rS | = | = peak response of the corresponding Standard for each of the known impurities from Standard solution 2 |
| CS | = | = concentration of the corresponding USP Reference Standard for each of the known impurities in Standard solution 2 (mg/mL) |
| CU | = | = concentration of Vinpocetine in the Sample solution (mg/mL) |
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of each of the unspecified impurities from the Sample solution |
| rS | = | = peak response of vinpocetine from Standard solution 2 |
| CS | = | = concentration of USP Vinpocetine RS in Standard solution 2 (mg/mL) |
| CU | = | = concentration of Vinpocetine in the Sample solution (mg/mL) |
Acceptance criteria:
See Impurity Table 1.
Impurity Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Vinpocetine | 1.00 | — |
| Vinpocetine related compound Aa |
0.40 | 0.6 |
| Vinpocetine related compound Bb |
0.75 | 0.5 |
| Vinpocetine related compound Cc |
0.83 | 0.3 |
| Vinpocetine related compound Dd |
0.68 | 0.5 |
| Unspecified individual impurity |
— | 0.1 |
| Total impurities | — | 1.0 |
|
a
Ethyl (12RS,13aSR,13bSR)-13a-ethyl-12-hydroxy-2,3,5,6,12,13,13a,13b-octahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylate (ethyl vincaminate).
b
Methyl (13aS,13bS)-13a-ethyl-9-methoxy-2,3,5,6,13a,13b-hexahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylate (apovincamine).
c
Ethyl (13aS,13bS)-13a-ethyl-10-methoxy-2,3,5,6,13a,13b-hexahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylate (methoxyvinpocetine).
d
Ethyl (12RS,13aRS,13bRS)-13a-ethyl-2,3,5,6,12,13,13a,13b-octahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylate (dihydrovinpocetine).
|
||
SPECIFIC TESTS
• Loss on Drying  731
731 :
Dry a sample in a vacuum at 100
:
Dry a sample in a vacuum at 100 for 3 h: NMT 0.5%.
for 3 h: NMT 0.5%.
• Optical Rotation, Specific rotation  781S
781S :
From +127.0
:
From +127.0 to +134.0
to +134.0 , determined at 20
, determined at 20 .
.
Sample solution:
10 mg/mL in dimethylformamide
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers, and store at controlled room temperature.
• USP Reference Standards  11
11
USP Vinpocetine Related Compound A RS
USP Vinpocetine Related Compound B RS
USP Vinpocetine Related Compound C RS
USP Vinpocetine Related Compound D RS
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Huy T. Dinh, M.S.
Scientific Liaison 1-301-816-8594 |
(DS2010) Monographs - Dietary Supplements |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1467
Pharmacopeial Forum: Volume No. 35(5) Page 1195