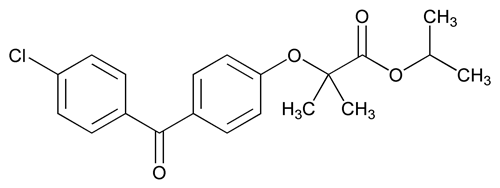
Fenofibrate
(fen'' oh fye' brate).
DEFINITION
Fenofibrate contains NLT 98.0% and NMT 102.0% of C20H21ClO4, calculated on the dried basis.
IDENTIFICATION
ASSAY
• Procedure
Mobile phase:
Acetonitrile and water acidified with phosphoric acid to a pH of 2.5 (7:3)
Standard solution:
1 mg/mL of USP Fenofibrate RS in Mobile phase
Sample solution:
1 mg/mL of Fenofibrate in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 286 nm
Column:
4.0-mm × 25-cm; packing L1
Flow rate:
1.0 mL/min
Injection size:
5 µL
System suitability
Sample:
Standard solution
Suitability requirements
Relative standard deviation:
NMT 1.0% for six replicate injections
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C20H21ClO4 in the portion of Fenofibrate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Fenofibrate RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Fenofibrate in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% on the dried basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.1%, determined on 1.0 g
:
NMT 0.1%, determined on 1.0 g
• Chloride and Sulfate, Chloride  221
221
Sample solution:
Add 25 mL of water to 5.0 g of Fenofibrate, and heat at 50 for 10 min. Cool, dilute with water to 50.0 mL, filter, and use the filtrate. [Note—Retain the remaining portion of the Sample solution for the test for Chloride and Sulfate, Sulfate. ]
for 10 min. Cool, dilute with water to 50.0 mL, filter, and use the filtrate. [Note—Retain the remaining portion of the Sample solution for the test for Chloride and Sulfate, Sulfate. ]
Analysis:
Use 10 mL of the Sample solution.
Acceptance criteria:
It shows no more chloride than corresponds to 0.15 mL of 0.020 N hydrochloric acid (0.01%).
• Chloride and Sulfate, Sulfate  221
221
Sample:
Use the Sample solution prepared in the test for Chloride and Sulfate, Chloride.
Analysis:
Use 10 mL of the Sample.
Acceptance criteria:
It shows no more sulfate than corresponds to 0.15 mL of 0.020 N sulfuric acid (0.01%).
• Heavy Metals, Method II  231
231 :
NMT 20 ppm
:
NMT 20 ppm
Organic Impurities
• Procedure
Mobile phase:
Acetonitrile and water acidified with phosphoric acid to a pH of 2.5 (7:3)
Impurity standard solution:
1 µg/mL each of USP Fenofibrate RS, USP Fenofibrate Related Compound A RS, and USP Fenofibrate Related Compound B RS, and 2 µg/mL of USP Fenofibrate Related Compound C RS in Mobile phase
Sample solution:
1 mg/mL of Fenofibrate in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 286 nm
Column:
4.0-mm × 25-cm; packing L1
Flow rate:
1.0 mL/min
Injection size:
20 µL
System suitability
Sample:
Impurity standard solution
Suitability requirements
Resolution:
NLT 1.5 between fenofibrate related compound A and fenofibrate related compound B
Analysis
Samples:
Impurity standard solution and Sample solution
Identify the fenofibrate peak and the peaks due to the impurities and degradation products listed in Impurity Table 1.
Measure the responses for the major peaks, and calculate the percentage of each of fenofibrate related compound A, fenofibrate related compound B, and fenofibrate related compound C in the portion of Fenofibrate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of appropriate fenofibrate related compound from the Sample solution |
| rS | = | = peak response of appropriate fenofibrate related compound from the Impurity standard solution |
| CS | = | = concentration of the appropriate fenofibrate related compound in the Impurity standard solution (µg/mL) |
| CU | = | = concentration of Fenofibrate in the Sample solution (µg/mL) |
Calculate the percentage of any other impurity in the portion of Fenofibrate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rS | = | = peak response of fenofibrate from the Impurity standard solution |
| CS | = | = concentration of fenofibrate in the Impurity standard solution (µg/mL) |
| CU | = | = concentration of Fenofibrate in the Sample solution (µg/mL) |
Acceptance criteria
Individual impurities:
See Impurity Table 1.
Total impurities:
NMT 0.5%
Impurity Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| (4-Chlorophenyl)(4-hydroxyphenyl) methanonea |
0.34 | 0.1 |
| 2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropanoic acid (fenofibric acid)b |
0.36 | 0.1 |
| (3RS)-3-[4-(4-Chlorobenzoyl) phenoxy]butan-2-one |
0.50 | 0.1 |
| Methyl 2-[4-(4-chlorobenzoyl) phenoxy]-2-methyl-propanoate |
0.65 | 0.1 |
| Ethyl 2-[4-(4-chlorobenzoyl) phenoxy]-2-methyl-propanoate |
0.80 | 0.1 |
| (4-Chlorophenyl)[4-(1-methylethoxy) phenyl]methanone |
0.85 | 0.1 |
| 1-Methylethyl 2-[[2-[4-(4-chlorobenzoyl)phenoxy]-2-methylpropanoyl]oxy]-2-methylpropanoatec | 1.35 | 0.2 |
| Any other impurity | — | 0.1 |
|
a
Fenofibrate related compound A.
b
Fenofibrate related compound B.
c
Fenofibrate related compound C.
|
||
SPECIFIC TESTS
• Acidity
Sample:
1.0 g
Analysis:
Dissolve the Sample in 50 mL of alcohol previously neutralized to phenolphthalein TS, and titrate with 0.1 N sodium hydroxide VS.
Acceptance criteria:
NMT 0.2 mL is required to change the color of the indicator to pink.
• Loss on Drying  731
731
Analysis:
Dry a sample in a vacuum over phosphorus pentoxide at 60 to constant weight.
to constant weight.
Acceptance criteria:
NMT 0.5%
• Color and Achromicity  631
631
Reference solution:
Mix 5 mL of Matching Fluid G and 95 mL of dilute hydrochloric acid (1 in 40).
Sample solution:
50 mg/mL of Fenofibrate in acetone
Analysis:
Proceed as directed in the chapter.
Acceptance criteria:
The Sample solution is not more intensely colored than the Reference solution.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed, light-resistant containers. Store at room temperature.
• USP Reference Standards  11
11
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Elena Gonikberg, Ph.D.
Principal Scientific Liaison 1-301-816-8251 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3160
Pharmacopeial Forum: Volume No. 35(2) Page 275