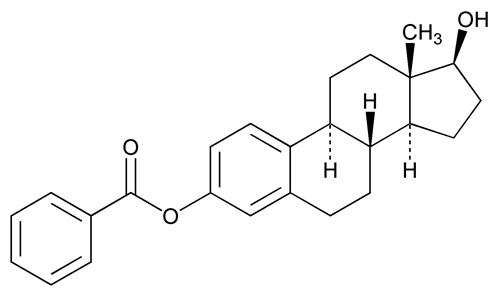
Estradiol Benzoate
Estra-1,3,5(10)-triene-3,17-diol, (17
Estradiol 3-benzoate
» Estradiol Benzoate contains not less than 97.0 percent and not more than 103.0 percent of C25H28O3, calculated on the dried basis.
Packaging and storage—
Preserve in tight, light-resistant containers.
Labeling—
Label it to indicate that it is for veterinary use only. Label it to indicate whether it is coarse grade or fine grade.
Identification—
B:
The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
Specific rotation  781S
781S :
between +57.0
:
between +57.0 and +63.0
and +63.0 .
.
Test solution:
10 mg per mL, previously dried, in dioxane.
Loss on drying—
Dry it at 100 to 105
to 105 for 3 hours: it loses not more than 0.5% of its weight.
for 3 hours: it loses not more than 0.5% of its weight.
Residue on ignition  281
281 :
not more than 0.2%, a 250-mg specimen being used.
:
not more than 0.2%, a 250-mg specimen being used.
Particle size  786
786 —
—
Suspension fluid—
To a mixture of glycerin and water (60:40, w/w) add a sufficient quantity of polysorbate 20 to obtain a solution having a concentration of 125 µL of polysorbate 20 per 100 g of solution.
Test suspension—
Saturate the Suspension fluid by adding about 100 mg of fine Estradiol Benzoate per 100 g of Suspension fluid, and sonicate for about 10 minutes. Filter the resulting suspension through a 0.45-µm nylon filter. To the filtrate, add about 50 mg of Estradiol Benzoate per mL of the filtered, saturated Suspension fluid, and mix on a vortex mixer until dispersed (about 1 minute).
Procedure—
Using a suitable multi-wavelength particle size analyzer,1 determine the particle size distribution within the Test suspension, analyzing the results in the range from 5 µm to 600 µm. Not more than 50% of the particles are less than 30 µm, and not less than 90% of the particles are less than 450 µm. The mean diameter of fine grade Estradiol Benzoate is not more than 100 µm, and the mean diameter of coarse grade Estradiol Benzoate is not less than 100 µm and not more than 200 µm.
Limit of methanol and dichloromethane—
Internal standard solution—
Prepare a solution that contains 0.1% (v/v) ethyl acetate in pyridine. [note—Inject 2 µL of the pyridine into the Chromatographic system to confirm that it contains no peaks that would interfere with the analysis. ]
Standard solution—
Transfer 50 µL of methylene chloride and 50 µL of methanol to a 50-mL volumetric flask, and dilute with Internal standard solution to volume. Mix well.
Test solution—
Accurately weigh about 100 mg of Estradiol Benzoate into a low-actinic glass vial. Dissolve in 1.0 mL of Internal standard solution, and mix.
Chromatographic system (see Chromatography  621
621 )—
The gas chromatograph is equipped with a flame-ionization detector and a 3.2-mm × 1.8-m stainless steel column packed with support S3. The injection port temperature is maintained at about 165
)—
The gas chromatograph is equipped with a flame-ionization detector and a 3.2-mm × 1.8-m stainless steel column packed with support S3. The injection port temperature is maintained at about 165 , the detector temperature is maintained at about 165
, the detector temperature is maintained at about 165 , and the column temperature is maintained at 140
, and the column temperature is maintained at 140 for 20 minutes, programmed thereafter to rise to 250
for 20 minutes, programmed thereafter to rise to 250 at a rate of 40
at a rate of 40 per minute, and then maintained at 250
per minute, and then maintained at 250 for 15 minutes. Helium is used as the carrier gas, flowing at a rate of about 40 mL per minute. Chromatograph the Internal standard solution and the Standard solution, and record the peak responses as directed for Procedure: the order of elution is methanol, methylene chloride, and ethyl acetate; no peaks are present within the Internal standard solution that would interfere with the integration of either the methanol or the methylene chloride peak; baseline resolution is achieved among the internal standard peak and residual solvent peaks; and the standard deviation for replicate injections of the Standard solution is not more than 5.0%.
for 15 minutes. Helium is used as the carrier gas, flowing at a rate of about 40 mL per minute. Chromatograph the Internal standard solution and the Standard solution, and record the peak responses as directed for Procedure: the order of elution is methanol, methylene chloride, and ethyl acetate; no peaks are present within the Internal standard solution that would interfere with the integration of either the methanol or the methylene chloride peak; baseline resolution is achieved among the internal standard peak and residual solvent peaks; and the standard deviation for replicate injections of the Standard solution is not more than 5.0%.
Procedure—
Separately inject equal volumes (about 2.0 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the peak areas for methanol, methylene chloride, and ethyl acetate. Calculate the percentage (w/w) of each residual solvent in the portion of Estradiol Benzoate taken by the formula:
100CI (DI / CU)(RU / RS)
in which CI is the concentration, in µL per mL, of the solvent of interest in the Standard solution; DI is the density, in g per mL, of the solvent of interest; CU is the concentration, in mg per mL, of Estradiol Benzoate in the Test solution; and RU and RS are the peak response ratios of the solvent of interest to the internal standard obtained from the Test solution and the Standard solution, respectively: the sum of the percentages of methanol and methylene chloride is not more than 0.20%.
Chromatographic purity—
Adsorbent:
a 0.25-mm layer of chromatographic silica gel mixture.
Developing solvent system—
Use a mixture of toluene and ethyl acetate (70:30).
Ammonium molybdate solution—
Dissolve 5 g of ammonium molybdate in 100 mL of 10% (v/v) sulfuric acid.
Diluent—
Prepare a solution containing a mixture of methylene chloride and alcohol (2:1).
Standard solution—
Dissolve an accurately weighed quantity of USP Estradiol Benzoate RS in Diluent to obtain a solution containing about 5 mg per mL.
Test solution 1—
Dissolve an accurately weighed quantity of Estradiol Benzoate in Diluent to obtain a solution containing about 5 mg per mL.
Test solution 2—
Transfer 200 µL of Test solution 1 to a 10-mL volumetric flask, and dilute with Diluent to volume.
Procedure (see Thin-Layer Chromatography under Chromatography  621
621 )—
Apply to the thin-layer chromatographic plate 20-µL aliquots of the Standard solution and Test solution 1 and 20-µL, 15-µL, 10-µL, 5-µL, and 2-µL aliquots of Test solution 2; the volumetric series of Test solution 2 represents 2.0%, 1.5%, 1.0%, 0.5% and 0.2% of the concentration of Estradiol Benzoate within the Test solution 1 spot. Allow the spots to dry, and develop the chromatogram until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and allow the solvent to evaporate from the plate. Spray the plate thoroughly with the Ammonium molybdate solution, and dry. Heat the plate in a drying oven for about 10 minutes at about 115
)—
Apply to the thin-layer chromatographic plate 20-µL aliquots of the Standard solution and Test solution 1 and 20-µL, 15-µL, 10-µL, 5-µL, and 2-µL aliquots of Test solution 2; the volumetric series of Test solution 2 represents 2.0%, 1.5%, 1.0%, 0.5% and 0.2% of the concentration of Estradiol Benzoate within the Test solution 1 spot. Allow the spots to dry, and develop the chromatogram until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and allow the solvent to evaporate from the plate. Spray the plate thoroughly with the Ammonium molybdate solution, and dry. Heat the plate in a drying oven for about 10 minutes at about 115 . Calculate the relative retardation factor, Rrel, (relative to estradiol benzoate) of all spots within the lanes for Test solution 1 and Test solution 2. Possible estradiol benzoate impurities include, but are not limited to, estradiol [estra-1,3,5(10)-triene-3,17b-diol], 17a-estradiol benzoate [estra-1,3,5(10)-triene-3,17a-diol 3-benzoate], and estrone [estra-1,3,5(10)-triene-17-one, 3-hydroxy]; their relative retardation factors, Rrel, are about 0.84, 1.15, and 1.21, respectively. Determine the percentages of each impurity by comparing the intensity of the impurity spots within Test solution 1 to those of the main spots obtained from the series of Test solution 2, ignoring any impurity peak less intense than the main spots found in the Test solution 2 lane containing 0.2% of the amount of estradiol benzoate of Test solution 1. Not more than 1.0% of any individual impurity is found, and not more than 2.0% of total impurities is found.
. Calculate the relative retardation factor, Rrel, (relative to estradiol benzoate) of all spots within the lanes for Test solution 1 and Test solution 2. Possible estradiol benzoate impurities include, but are not limited to, estradiol [estra-1,3,5(10)-triene-3,17b-diol], 17a-estradiol benzoate [estra-1,3,5(10)-triene-3,17a-diol 3-benzoate], and estrone [estra-1,3,5(10)-triene-17-one, 3-hydroxy]; their relative retardation factors, Rrel, are about 0.84, 1.15, and 1.21, respectively. Determine the percentages of each impurity by comparing the intensity of the impurity spots within Test solution 1 to those of the main spots obtained from the series of Test solution 2, ignoring any impurity peak less intense than the main spots found in the Test solution 2 lane containing 0.2% of the amount of estradiol benzoate of Test solution 1. Not more than 1.0% of any individual impurity is found, and not more than 2.0% of total impurities is found.
Assay—
Mobile phase—
Prepare a filtered and degassed mixture of acetonitrile and water (7:3). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
System suitability preparation—
Transfer an accurately weighed quantity of about 20.0 mg each of USP Estradiol Benzoate RS and estradiol 17-acetate into a 100-mL volumetric flask. Add 70 mL of acetonitrile, and sonicate until dissolved. Add 25 mL of water, mix well, and allow to equilibrate to ambient temperature. Dilute with water to volume, and mix.
Standard preparation—
Transfer an accurately weighed quantity of about 20.0 mg of USP Estradiol Benzoate RS into a 100-mL volumetric flask. Add 70 mL of acetonitrile, and sonicate until dissolved. Add 25 mL of water, mix well, and allow to equilibrate to ambient temperature. Dilute with water to volume, and mix.
Assay preparation—
Transfer an accurately weighed quantity of about 20.0 mg of Estradiol Benzoate into a 100-mL volumetric flask. Add 70 mL of acetonitrile, and sonicate until dissolved. Add 25 mL of water, mix well, and allow to equilibrate to ambient temperature. Dilute with water to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 230-nm detector, a 4.6-mm × 4.5-cm guard column that contains packing L1, and a 4.6-mm × 25-cm analytical column that contains 5-µm packing L1. The flow rate is about 1.5 mL per minute. Chromatograph the System suitability preparation and the Standard preparation, and record the peak responses as directed for Procedure: the relative retention times are about 0.5 for estradiol 17-acetate and 1.0 for estradiol benzoate; the resolution, R, between estradiol 17-acetate and estradiol benzoate is not less than 6.0; the column efficiency is not less than 8000 theoretical plates for estradiol benzoate; the tailing factor for estradiol benzoate is not more than 2.0; and, using the Standard preparation, the relative standard deviation for replicate injections is not more than 1.5%.
)—
The liquid chromatograph is equipped with a 230-nm detector, a 4.6-mm × 4.5-cm guard column that contains packing L1, and a 4.6-mm × 25-cm analytical column that contains 5-µm packing L1. The flow rate is about 1.5 mL per minute. Chromatograph the System suitability preparation and the Standard preparation, and record the peak responses as directed for Procedure: the relative retention times are about 0.5 for estradiol 17-acetate and 1.0 for estradiol benzoate; the resolution, R, between estradiol 17-acetate and estradiol benzoate is not less than 6.0; the column efficiency is not less than 8000 theoretical plates for estradiol benzoate; the tailing factor for estradiol benzoate is not more than 2.0; and, using the Standard preparation, the relative standard deviation for replicate injections is not more than 1.5%.
Procedure—
Separately inject equal volumes (about 20 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of C25H28O3 in the portion of Estradiol Benzoate taken by the formula:
100C(rU / rS)
in which C is the concentration, in mg per mL, of USP Estradiol Benzoate RS in the Standard preparation; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
1
A suitable multi-wavelength particle size analyzer is model LS 13 320, obtained from Beckman Coulter, Inc., Fullerton, CA, or equivalent.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Morgan Puderbaugh, B.S.
Associate Scientific Liaison 1-301-998-6833 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3100
Pharmacopeial Forum: Volume No. 33(2) Page 229