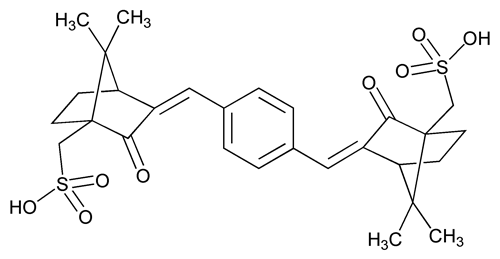
Ecamsule Solution
Bicyclo[2.2.1]heptane-1-methanesulfonic acid, 3,3¢-(1,4-phenylenedimethylidyne)bis[7,7-dimethyl-2-oxo]-.
(±)-(3E,3¢E)-3,3¢-(p-Phenylenedimethylidyne)bis[2-oxo-10-bornanesulfonic acid]
» Ecamsule Solution is an aqueous solution of C28H34O8S2. It contains not less than 30.0 percent and not more than 34.0 percent, by weight, of ecamsule (C28H34O8S2).
Packaging and storage—
Preserve in tight containers. Protect from light, and store at room temperature.
USP Reference standards  11
11 —
—
USP Ecamsule Related Compound A RS
1,4-Phenylenedimethanol.
C8H10O2 138.16
1,4-Phenylenedimethanol.
C8H10O2 138.16
USP Ecamsule Related Compound B RS
4-(Hydroxymethyl)benzoic acid.
C8H8O3 152.15
4-(Hydroxymethyl)benzoic acid.
C8H8O3 152.15
USP Ecamsule Related Compound C RS
Terephthalic acid.
C8H6O4 166.13
Terephthalic acid.
C8H6O4 166.13
USP Ecamsule Related Compound D RS
((1SR,4RS)-7,7-Dimethyl-2-oxobicyclo[2.2.1]heptan-1-yl)methanesulfonic acid.
C10H16O4S 232.30
((1SR,4RS)-7,7-Dimethyl-2-oxobicyclo[2.2.1]heptan-1-yl)methanesulfonic acid.
C10H16O4S 232.30
USP Ecamsule Related Compound E RS
Sodium ((1SR,4SR,E)-3-(4-(hydroxymethyl)benzylidene)-7,7-dimethyl-2-oxobicyclo[2.2.1]heptan-1-yl)methanesulfonate.
C18H21NaO5S 372.41
Sodium ((1SR,4SR,E)-3-(4-(hydroxymethyl)benzylidene)-7,7-dimethyl-2-oxobicyclo[2.2.1]heptan-1-yl)methanesulfonate.
C18H21NaO5S 372.41
USP Ecamsule Related Compound F RS
4-((E)-((1SR,4SR)-7,7-Dimethyl-3-oxo-4-(sulfomethyl)bicyclo[2.2.1]heptan-2-ylidene)methyl)benzoic acid.
C18H20O6S 364.41
4-((E)-((1SR,4SR)-7,7-Dimethyl-3-oxo-4-(sulfomethyl)bicyclo[2.2.1]heptan-2-ylidene)methyl)benzoic acid.
C18H20O6S 364.41
USP Ecamsule Related Compound G RS
4-((E)-((1SR,4SR)-7,7-Dimethyl-3-oxo-4-(sulfomethyl)bicyclo[2.2.1]heptan-2-ylidene)methyl)benzaldehyde, sodium salt.
C18H19NaO5S 370.40
4-((E)-((1SR,4SR)-7,7-Dimethyl-3-oxo-4-(sulfomethyl)bicyclo[2.2.1]heptan-2-ylidene)methyl)benzaldehyde, sodium salt.
C18H19NaO5S 370.40
USP Ecamsule Triethanolamine RS
Bicyclo[2.2.1]heptane-1-methanesulfonic acid, 3,3¢-(1,4-phenylenedimethylidyne)bis[7,7-dimethyl-2-oxo]-, ditriethanolamine salt (1:2).
C28H34O8S2·(C6H15NO3)2 861.07
Bicyclo[2.2.1]heptane-1-methanesulfonic acid, 3,3¢-(1,4-phenylenedimethylidyne)bis[7,7-dimethyl-2-oxo]-, ditriethanolamine salt (1:2).
C28H34O8S2·(C6H15NO3)2 861.07
Labeling—
The label states that this article is not intended for direct administration to humans or animals.
Identification—
A:
Infrared Absorption  197A
197A —Place a drop of Ecamsule Solution on a diamond sampling surface and dry it with a stream of warm air. The IR absorption spectrum conforms to that of USP Ecamsule Solution RS, similarly obtained.
—Place a drop of Ecamsule Solution on a diamond sampling surface and dry it with a stream of warm air. The IR absorption spectrum conforms to that of USP Ecamsule Solution RS, similarly obtained.
B:
Ultraviolet Absorption  197U
197U —
—
Solution—
Transfer 0.25 g of Ecamsule Solution to a 100-mL volumetric flask, and dilute with water to volume. Further dilute 2 mL of this solution with water to 100 mL.
The Solution exhibits absorption maximum between 342 and 346 nm.
Limit of chloride—
Dissolve about 10 g of Ecamsule Solution, accurately weighed, in 70 mL of water. Titrate this solution with 0.01 N silver nitrate, determine the endpoint potentiometrically (see Titrimetry  541
541 ), and calculate the percentage of chloride in the portion of C28H34O8S2 taken by the formula:
), and calculate the percentage of chloride in the portion of C28H34O8S2 taken by the formula:
100(35.5)(VN/W)(100/A)
in which 35.5 is the atomic weight, in g per mole, of chloride; V is the volume, in mL, of silver nitrate used for titration; N is the concentration, in normality, of silver nitrate; W is the weight, in mg, of Ecamsule Solution taken for determination; and A is the assay, in percent, of Ecamsule Solution: not more than 0.3% of chloride is found.
Limit of sodium—
Diluent—
Transfer 5 mL of nitric acid in a 1000-mL volumetric flask containing about 500 mL of water, and dilute with water to volume.
Test solution—
Transfer about 1 g of Ecamsule Solution, accurately weighed, to a 100-mL volumetric flask, and dilute with Diluent to volume.
Standard solutions—
Dilute quantitatively, and stepwise if necessary, a commercially available sodium atomic absorption standard solution containing 1000 µg of sodium per mL with Diluent to obtain solutions having known concentrations of 1, 5, 10, and 20 µg per mL, respectively.
Procedure (see Spectrophotometry and Light Scattering  851
851 )—
Concomitantly determine the absorbances of the Standard solutions and the Test solution at the sodium emission line of 330 nm or 589 nm with a suitable atomic absorption spectrophotometer equipped with a sodium lamp and an air–acetylene flame, using Diluent as the blank. Determine the concentration of sodium, in µg per mL, in the Test solution using the calibration graph. Calculate the percentage of sodium in the portion of C28H34O8S2 taken by the formula:
)—
Concomitantly determine the absorbances of the Standard solutions and the Test solution at the sodium emission line of 330 nm or 589 nm with a suitable atomic absorption spectrophotometer equipped with a sodium lamp and an air–acetylene flame, using Diluent as the blank. Determine the concentration of sodium, in µg per mL, in the Test solution using the calibration graph. Calculate the percentage of sodium in the portion of C28H34O8S2 taken by the formula:
100 × 10–6(CV/W)(100/A)
in which C is the concentration, in µg per mL, of sodium in the Test solution, the multiplier of 10–6 is for conversion of µg per mL to g per mL; V is the volume, in mL, of Test solution; W is the weight, in g, of Ecamsule Solution taken for determination; and A is the assay, in percent, of Ecamsule Solution: not more than 0.3% of sodium is found.
Related compounds—
Test for related compounds A to F—
Solvent A—
Prepare a mixture of acetonitrile and 85% phosphoric acid (1000:1).
Solvent B—
Prepare a mixture of water and 85% phosphoric acid (1000:1).
Standard solution—
Dissolve an accurately weighed quantity of USP Ecamsule Related Compound A RS, USP Ecamsule Related Compound B RS, USP Ecamsule Related Compound C RS, USP Ecamsule Related Compound D RS, USP Ecamsule Related Compound E RS, and USP Ecamsule Related Compound F RS in water, sonicating if necessary, to obtain a solution having known concentrations as found in Table 1.
Test solution—
Transfer about 100 mg, accurately weighed, of Ecamsule Solution to a 50-mL volumetric flask, and dilute with water to volume.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with either a programmable variable wavelength detector or two separate detectors capable of monitoring at 200 nm and 300 nm and a 4.6-mm × 15-cm column that contains 5-µm packing L1. The flow rate is about 1 mL per minute. The chromatograph is programmed as follows.
)—
The liquid chromatograph is equipped with either a programmable variable wavelength detector or two separate detectors capable of monitoring at 200 nm and 300 nm and a 4.6-mm × 15-cm column that contains 5-µm packing L1. The flow rate is about 1 mL per minute. The chromatograph is programmed as follows.
Chromatograph the Standard solution, and record the peak areas as directed for Procedure: the relative standard deviations of the ecamsule related compound peaks for replicate injections are not more than 10.0%; and the resolution, R, between all adjacent peak pairs of ecamsule related compounds is not less than 1.5 measured at 200 nm.
| Time (minutes) |
Solution A
(%) |
Solution B
(%) |
Elution |
|---|---|---|---|
| 0–25 | 20 | 80 | isocratic |
| 25–27 | 20®80 | 80®20 | linear gradient |
| 27–47 | 80 | 20 | isocratic |
| 47–50 | 80®20 | 20®80 | linear gradient |
| 50–65 | 20 | 80 | equilibration |
Procedure—
Separately inject equal volumes (about 10 µL) of the Standard solution and the Test solution into the chromatograph, allow the chromatogram to run for about 20 minutes for the Standard solution and 60 minutes for the Test solution, record the chromatograms at 200 nm from 0 to 8 minutes and at 300 nm after 8 minutes, and measure the peak areas. Calculate the percentage of ecamsule related compounds A, B, C, D, and F in the portion of C28H34O8S2 taken by the formula:
100[100CS / (CU × A)](rU / rS)
in which CS is the concentration, in mg per mL, of the ecamsule related compound in the Standard solution; CU is the concentration, in mg per mL, of the Test solution; A is the assay, in percent, obtained from the Assay; and rU and rS are the peak areas of the ecamsule related compound obtained from the Test solution and Standard solution, respectively. Calculate the percentage of ecamsule related compound E in the portion of C28H34O8S2 taken by the formula:
100(350.43/372.41)[100CS / (CU × A)](rU / rS)
in which 350.43 and 372.41 are the molecular weights of ecamsule related compound E (free acid) and USP Ecamsule Related Compound E RS (sodium salt), respectively; CS is the concentration, in mg per mL, of ecamsule related compound E in the Standard solution; CU is the concentration, in mg per mL, of the Test solution; A is the assay, in percent, obtained from the Assay; and rU and rS are the peak areas of the ecamsule related compound E obtained from the Test solution and Standard solution, respectively. The limits are given in Table 1.
Table 1
| Name | Concentration (mg/mL) in the Standard solution |
Detection wavelength (nm) |
RRT1 | Limit (%) |
|
|---|---|---|---|---|---|
| Ecamsule related compound A | 0.001 | 200 | 0.42 | 0.2 | |
| Ecamsule related compound B | 0.001 | 200 | 0.54 | 0.2 | |
| Ecamsule related compound C | 0.001 | 200 | 0.70 | 0.2 | |
| Ecamsule related compound D | 0.008 | 200 | 1.00 | 1.3 | |
| Ecamsule related compound E (free acid) | 0.004 | 300 | 2.52 | 0.7 | |
| Ecamsule related compound F | 0.004 | 300 | 3.26 | 0.7 | |
|
1
Ecamsule elutes after 27 minutes and is a broad peak in the Test for related compounds A to F. The relative retention times of related compounds are measured with respect to ecamsule related compound D.
|
|||||
Test for related compound G, Ecamsule exo-2-hydroxyecamsule, Ecamsule endo-2-hydroxyecamsule, and unspecified impurities—
Mobile phase—
Proceed as directed in the Assay.
Standard solution 1—
Dissolve an accurately weighed quantity of USP Ecamsule Related Compound G RS in water to obtain a solution having a known concentration of about 0.005 mg per mL.
Standard solution 2—
Use the Standard preparation, as described in the Assay.
Test solution—
Use the Assay preparation, prepared as directed in the Assay.
Chromatographic system—
Prepared as directed in the Assay. Use the liquid chromatograph equipped with a 310-nm detector in addition to using a 343-nm detector. [note—Ecamsule related compound G is detected at 310 nm; and Ecamsule exo-2-hydroxyecamsule, Ecamsule endo-2-hydroxyecamsule, and unspecified impurities are detected at 343 nm. ]
Procedure—
Separately inject equal volumes (about 20 µL) of Standard solution 1, Standard solution 2, and the Test solution into a chromatograph, record the chromatograms for not less than 6 times the retention time of ecamsule trans-trans isomer, and measure the peak areas. Calculate the percentage of ecamsule related compound G in the portion of C28H34O8S2 taken by the formula:
100(348.41/370.40)[100CS / (CU × A)](rU / rS)
in which 348.41 and 370.40 are the molecular weights of ecamsule related compound G and USP Ecamsule Related Compound G RS, respectively; CS is the concentration, in mg per mL, of USP Ecamsule Related Compound G RS in Standard solution 1; CU is the concentration, in mg per mL, of the Test solution; A is the assay, in percent, obtained from the Assay; and rU and rS are the peak areas of ecamsule related compound G obtained from the Test solution and Standard solution 1, respectively. Calculate the percentage of Ecamsule exo-2-hydroxyecamsule and Ecamsule endo-2-hydroxyecamsule in the portion of C28H34O8S2 taken by the formula:
100(1/F)[100CS / (CU × A)](ri / rS)
in which F is the relative response factor for each impurity obtained from Table 2; CS is the concentration, in mg per mL, of USP Ecamsule Triethanolamine RS in Standard solution 2; CU is the concentration, in mg per mL, of the Test solution; A is the assay, in percent, obtained from the Assay; ri is the peak area for each impurity obtained from the Test solution; and rS is the sum of peak areas corresponding to the trans-trans and cis-trans isomers obtained from the Standard solution. Calculate the percentage of any unspecified impurity in the portion of C28H34O8S2 taken by the formula:
100(ri / rS)
in which ri is the peak area for each unspecified impurity obtained from the Test solution; and rS is the sum of all peak areas obtained from the Test solution. The limits are given in Table 2.
Table 2
| Name | RRT3 | F | Limit (%) |
|---|---|---|---|
| Ecamsule related compound G | 0.9 | — | 0.2 |
| Ecamsule exo-2-hydroxyecamsule1 | 1.4 | 0.6 | 0.2 |
| Ecamsule endo-2-hydroxyecamsule2 | 1.6 | 0.6 | 0.3 |
| Any single unspecified impurity | — | 1.0 | 0.5 |
|
1
[(1SR,2R,4SR,E)-3-(4-{(E)-[(1SR,4SR)-7,7-dimethyl-3-oxo-4-(sulfomethyl)bicyclo[2.2.1]heptan-2-ylidene]methyl}benzylidene)-2-hydroxy-7,7-dimethylbicyclo[2.2.1]heptan-1-yl]methanesulfonic acid [C28H36O8S2, 564.71].
2
[(1SR,2S,4SR,E)-3-(4-{(E)-[(1SR,4SR)-7,7-dimethyl-3-oxo-4-(sulfomethyl)bicyclo[2.2.1]heptan-2-ylidene]methyl}benzylidene)-2-hydroxy-7,7-dimethylbicyclo[2.2.1]heptan-1-yl]methanesulfonic acid [C28H36O8S2, 564.71].
3
The relative retention times are measured with respect to ecamsule trans-trans isomer.
|
|||
Total impurities—
Calculate the sum of the related compounds and unspecified impurities from the Test for related compounds A to F and the Test for related compound G, Ecamsule exo-2-hydroxyecamsule, Ecamsule endo-2-hydroxyecamsule, and unspecified impurities: not more than 5.0% of total impurities is found.
Assay—
[note—Prepare solutions immediately before use, and protect them from light in low-actinic glassware. ]
1% Triethylamine solution—
Prepare a mixture of water and triethylamine (100:1), and adjust with phosphoric acid to a pH of 7.
Mobile phase—
Prepare a mixture of 1% Triethylamine solution and methanol (50:50). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard preparation—
Dissolve an accurately weighed quantity of USP Ecamsule Triethanolamine RS in Mobile phase, sonicating if necessary, to obtain a solution having a known concentration of about 0.12 mg per mL of ecamsule triethanolamine.
Assay preparation—
Transfer about 500 mg, accurately weighed, of Ecamsule Solution to a 100-mL volumetric flask, and dilute with water to volume. Transfer 5.0 mL of this solution into a 100-mL volumetric flask, and dilute with water to volume.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 343-nm detector and a 4.0-mm × 125-mm column that contains 5-µm packing L1. The flow rate is about 1 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative retention times are about 1.0 for ecamsule trans-trans isomer and 2.9 for the ecamsule cis-trans isomer; the relative standard deviation of the sum of the ecamsule trans-trans and cis-trans peak areas for replicate injections is not more than 2.0%; and the number of theoretical plates of the peak corresponding to the trans-trans isomer is not less than 1430.
)—
The liquid chromatograph is equipped with a 343-nm detector and a 4.0-mm × 125-mm column that contains 5-µm packing L1. The flow rate is about 1 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative retention times are about 1.0 for ecamsule trans-trans isomer and 2.9 for the ecamsule cis-trans isomer; the relative standard deviation of the sum of the ecamsule trans-trans and cis-trans peak areas for replicate injections is not more than 2.0%; and the number of theoretical plates of the peak corresponding to the trans-trans isomer is not less than 1430.
Procedure—
Inject equal volumes (about 20 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the peak areas. Calculate the percentage of C28H34O8S2 in the portion of Ecamsule Solution taken by the formula:
100(562.69/861.07)(V/W) C (rU / rS)
in which 562.69 and 861.07 are the molecular weights of ecamsule and ecamsule triethanolamine, respectively; V is the volume, in mL, of the Assay preparation; W is the weight, in mg, of Ecamsule Solution used for the Assay preparation; C is the concentration, in mg per mL, of ecamsule triethanolamine in the Standard preparation; and rU and rS are the sum of peak areas corresponding to trans-trans and cis-trans isomers obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Feiwen Mao, M.S.
Senior Scientific Liaison 1-301-816-8320 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3003
Pharmacopeial Forum: Volume No. 34(5) Page 1153