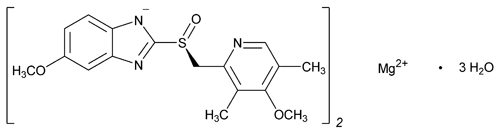
Esomeprazole Magnesium
C34H36MgN6O6S2·3H2O Trihydrate: 767.17
C34H36MgN6O6S2 Anhydrous: 713.12
1H-Benzimidazole,5-methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl], magnesium salt (2:1), trihydrate;
5-Methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]sulfinyl]benzimidazole, magnesium salt (2:1), trihydrate

 [217087-09-7].
[217087-09-7].
(es'' oh mep' ra zole mag nee' zee um).
C34H36MgN6O6S2·3H2O Trihydrate: 767.17
C34H36MgN6O6S2 Anhydrous: 713.12
1H-Benzimidazole,5-methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl], magnesium salt (2:1), trihydrate;
5-Methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]sulfinyl]benzimidazole, magnesium salt (2:1), trihydrate
DEFINITION
Esomeprazole Magnesium contains NLT 98.0% and NMT 102.0% of C34H36MgN6O6S2, calculated on the anhydrous basis.
IDENTIFICATION
• B.
The Sample solution, prepared and tested as directed in the test for Content of Magnesium, exhibits a significant absorption at 285.2 nm.
ASSAY
• Procedure
Solution A:
Dissolve 0.725 g of monobasic sodium phosphate and 4.472 g of anhydrous dibasic sodium phosphate in 300 mL of water, and dilute with water to 1000 mL. Dilute 250 mL of this solution with water to 1000 mL. If necessary, adjust with phosphoric acid to a pH of 7.6.
Solution B:
Mix 11 mL of 0.25 M tribasic sodium phosphate with 22 mL of 0.5 M dibasic sodium phosphate, and dilute with water to 100 mL.
Mobile phase:
Acetonitrile and Solution A (7:13)
Standard solution:
Transfer 10 mg of USP Omeprazole RS to a 200-mL volumetric flask, and dissolve in about 10 mL of methanol. Add 10 mL of Solution B, and dilute with water to volume. [Note—This solution contains 0.05 mg/mL of omeprazole. ]
Sample solution:
Transfer 10 mg of Esomeprazole Magnesium to a 200-mL volumetric flask, and dissolve in about 10 mL of methanol. Add 10 mL of Solution B, and dilute with water to volume. [Note—This solution contains 0.05 mg/mL of esomeprazole magnesium. ]
Chromatographic system
Mode:
LC
Detector:
UV 280 nm
Column:
4.0-mm × 12.5-cm or a 4.6-mm × 15-cm; 5-µm packing L7. [Note—Alternatively, a 3.9-mm × 15-cm column that contains 4-µm packing L1 may be used. ]
Flow rate:
1 mL/min
Injection size:
20 µL
System suitability
Sample:
Standard solution
Suitability requirements
Column efficiency:
NLT 2000 theoretical plates
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C34H36MgN6O6S2 in the portion of Esomeprazole Magnesium taken:
Result = (rU/rS) × (CS/CU) × [Mr1/(2 × Mr2)] × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of omeprazole in the Standard solution (mg/mL) |
| CU | = | = concentration of Esomeprazole Magnesium in the Sample solution (mg/mL) |
| Mr1 | = | = molecular weight of esomeprazole magnesium, 713.12 |
| Mr2 | = | = molecular weight of omeprazole, 345.42 |
Acceptance criteria:
98.0%–102.0% on the anhydrous basis
OTHER COMPONENTS
• Content of Magnesium
Lanthanum solution:
Transfer 58.7 g of lanthanum oxide into a 1000-mL volumetric flask, wet the substance with some water, and dissolve by cautious addition of 250 mL of hydrochloric acid in 20- to 30-mL portions, cooling between the additions. Add water while stirring, cool to room temperature, and dilute with water to volume. [Note—Store the solution in a plastic bottle. ]
Standard stock solution:
1000 µg/mL of magnesium in water, from a commercially prepared atomic absorption standard solution. [Note—Store the solution in a plastic bottle. ]
Standard solution A:
Transfer 10.0 mL of Standard stock solution to a 500-mL volumetric flask, add 50 mL of 1 N hydrochloric acid, and dilute with water to volume. Transfer 20.0 mL of this solution to a 200-mL volumetric flask, and dilute with water to volume. [Note—This solution contains 2 µg/mL of magnesium. ]
Standard solution B:
Combine 5.0 mL of Standard solution A and 4.0 mL of Lanthanum solution, and dilute with water to 100.0 mL (0.1 µg/mL).
Standard solution C:
Combine 10.0 mL of Standard solution A and 4.0 mL of Lanthanum solution, and dilute with water to 100.0 mL (0.2 µg/mL).
Standard solution D:
Combine 15.0 mL of Standard solution A and 4.0 mL of Lanthanum solution, and dilute with water to 100.0 mL (0.3 µg/mL).
Standard solution E:
Combine 20.0 mL of Standard solution A and 4.0 mL of Lanthanum solution, and dilute with water to 100.0 mL (0.4 µg/mL).
Standard solution F:
Combine 25.0 mL of Standard solution A and 4.0 mL of Lanthanum solution, and dilute with water to 100.0 mL (0.5 µg/mL). [Note—Concentrations of the Standard solutions and the Sample solution may be modified to fit the linear or working range of the instrument. When using instruments with a linear calibration graph, the number of Standard solutions can be reduced. ]
Blank solution:
Transfer 4.0 mL of Lanthanum solution to a 100-mL volumetric flask, and dilute with water to volume.
Sample solution:
Transfer 250 mg of Esomeprazole Magnesium to a 100-mL volumetric flask, add 20 mL of 1 N hydrochloric acid, swirl until dissolved, and dilute with water to volume. Allow to stand for 30 min. Transfer 10.0 mL of this solution to a 200-mL volumetric flask, and dilute with water to volume. Transfer 10.0 mL of the solution to another 100-mL volumetric flask, add 4.0 mL of Lanthanum solution, and dilute with water to volume.
Spectrometric conditions
Mode:
Atomic absorption spectrophotometer
Flame:
Air–acetylene
Analytical wavelength:
285.2 nm
Analysis
Samples:
Standard solution B, Standard solution C, Standard solution D, Standard solution E, Standard solution F, Blank solution, and Sample solution
Determine the concentration, Cs, in µg/mL, of magnesium in the Sample solution using the calibration graph.
Calculate the percentage of magnesium in the portion of Esomeprazole Magnesium taken:
Result = (CS/CU) × (100/(100  F)) × 100
F)) × 100
| CS | = | = content of magnesium in the Sample solution as calculated above (µg/mL) |
| CU | = | = concentration of Esomeprazole Magnesium in the Sample solution (µg/mL) |
| F | = | = content of water in Esomeprazole Magnesium, as determined in Specific Tests, Water Determination (%) |
Acceptance criteria:
3.30%–3.55%, on anhydrous basis
IMPURITIES
Organic Impurities
• Procedure 1
Solution A:
0.725 g of monobasic sodium phosphate and 4.472 g of anhydrous dibasic sodium phosphate in 300 mL of water, and dilute with water to 1000 mL. Dilute 250 mL of this solution with water to 1000 mL. If necessary, adjust with phosphoric acid to a pH of 7.6.
Mobile phase:
Acetonitrile and Solution A (11:29). [Note—To improve the resolution, the composition may be changed to 1:3, if necessary. ]
System suitability solution:
1 mg of USP Omeprazole RS and 1 mg of USP Omeprazole Related Compound A RS in 25 mL of Mobile phase. [Note—Omeprazole Related Compound A is omeprazole sulfone. ]
Sample solution:
4 mg of Esomeprazole Magnesium in 25 mL of Mobile phase. [Note—Prepare this solution fresh. ]
Chromatographic system
Mode:
LC
Detector:
UV 280 nm
Column:
4.0-mm × 12.5-cm or a 4.6-mm × 15-cm; 5-µm packing L7. [Note—Alternatively, a 3.9-mm × 15-cm column that contains 4-µm packing L1 may be used. ]
Flow rate:
0.8–1 mL/min
Injection size:
50 µL
System suitability
Suitability requirements
Resolution:
NLT 3 between omeprazole related compound A and omeprazole
Analysis
Sample:
Sample solution
Record the chromatogram for at least 4.5 times the retention time of the omeprazole peak, and measure the peak responses. Identify the impurities based on the retention times shown in Impurity Table 1.
Calculate the percentage of any individual impurity in the portion of Esomeprazole Magnesium taken:
Result = (rU/rT) × 100
| rU | = | = peak response for each impurity |
| rT | = | = sum of all peak responses |
Acceptance criteria
Individual impurities:
See Impurity Table 1.
Total impurities:
NMT 0.5%
Impurity Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Omeprazole N-oxide a | 0.45 | 0.1 |
| Omeprazole sulfoneb (related compound A) | 0.8 | 0.2 |
| Any other individual impurities | — | 0.1 |
| Omeprazole | 1.0 | — |
|
a
4-Methoxy-2-[[(RS)-(5-methoxy-1H-benzimidazol-2-yl)sulfinyl]methyl]-3,5-dimethylpyridine 1-oxide.
b
5-Methoxy-2-[[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfonyl]-1H-benzimidazole.
|
||
• Procedure 2: Enantiomeric Purity
Solution A:
Mix 70 mL of 1 M monobasic sodium phosphate with 20 mL of 0.5 M dibasic sodium phosphate, and dilute with water to 1000 mL. Dilute 250 mL of this solution with water to 1000 mL.
Diluent:
Mix 11 mL of 0.25 M tribasic sodium phosphate with 22 mL of 0.5 M dibasic sodium phosphate, and dilute with water to 1000 mL.
Mobile phase:
Acetonitrile and Solution A (3:17)
System suitability solution:
2 mg of USP Omeprazole RS in 10 mL of Diluent. Dilute 1.0 mL of this solution with Diluent to 50 mL.
Sample solution:
40 mg of Esomeprazole Magnesium in 5 mL of methanol, and dilute with Diluent to 25 mL. Dilute 1 mL of this solution with Diluent to 50 mL.
Chromatographic system
Mode:
LC
Detector:
UV 302 nm
Column:
4.0-mm × 10-cm; packing L41
Flow rate:
0.6 mL/min
Injection size:
20 µL
System suitability
Sample:
System suitability solution
Suitability requirements
Resolution:
NLT 3 between the enantiomer peaks. [Note—The elution order is the R-enantiomer, followed by the esomeprazole peak, which is the S-enantiomer. ]
Analysis
Sample:
Sample solution
Calculate the percentage of the R-enantiomer in the portion of Esomeprazole Magnesium taken:
Result = (rU/rS) × 100
| rU | = | = peak response for the R-enantiomer |
| rS | = | = sum of the peak responses for esomeprazole and R-enantiomer |
Acceptance criteria:
NMT 0.2% of the R-enantiomer
SPECIFIC TESTS
• Water Determination, Method I  921
921 :
6.0%–8.0%
:
6.0%–8.0%
• Color of Solution
Sample solution:
20 mg/mL of Esomeprazole Magnesium in methanol
Analysis:
Determine the absorbance of this solution at 440 nm, in 1-cm cells, using methanol as the blank.
Acceptance criteria:
NMT 0.2
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers, protected from light. Store at room temperature.
• USP Reference Standards  11
11
USP Omeprazole Related Compound A RS
Omeprazole sulfone, 5-methoxy-2-[[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfonyl]-1H-benzimidazole.
C17H19N3O4S 361.42

 [CAS-88546-55-8].
[CAS-88546-55-8].
Omeprazole sulfone, 5-methoxy-2-[[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfonyl]-1H-benzimidazole.
C17H19N3O4S 361.42
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Elena Gonikberg, Ph.D.
Principal Scientific Liaison 1-301-816-8251 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3097
Pharmacopeial Forum: Volume No. 35(3) Page 550