Enoxaparin Sodium
(ee nox ap' a rin soe' dee um; ee nox'' a par' in soe' dee um).
DEFINITION
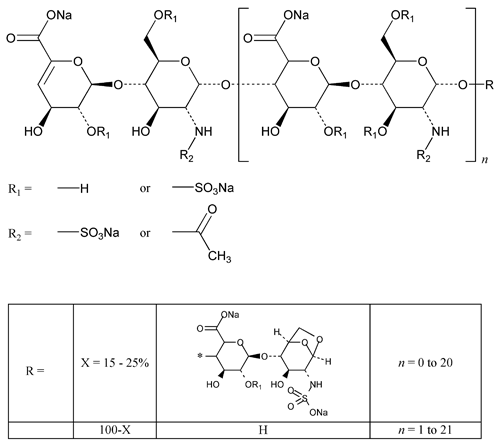
Enoxaparin Sodium is the sodium salt of a depolymerized heparin. It is obtained by alkaline depolymerization of heparin benzyl ester. The starting material, heparin, is obtained exclusively from porcine intestinal mucosa. Heparin source material used in the manufacture of Enoxaparin Sodium complies with the compendial requirements stated in the Heparin Sodium monograph. Enoxaparin Sodium consists of a complex set of oligosaccharides that have not yet been completely characterized. The majority of the components have a 4-enopyranose uronate structure at the nonreducing end of their chain. About 20% of the materials contain a 1,6-anhydro derivative on the reducing end of the chain, the range being between 15% and 25%. The weight-average molecular weight of Enoxaparin Sodium is 4500 Da, the range being between 3800 and 5000 Da; about 16% have a molecular weight of less than 2000 Da, the range being between 12.0% and 20.0%; about 74% have a molecular weight between 2000 and 8000 Da, the range being between 68.0% and 82.0%. NMT 18.0% have a molecular weight higher than 8000 Da. When prepared as a solution, the solution is analyzed for clarity and degree of color using a validated method. The degree of sulfation is NLT 1.8 per disaccharide unit. It has a potency of NLT 90 and NMT 125 Anti-Factor Xa International Units (IU)/mg, and NLT 20.0 and NMT 35.0 Anti-Factor IIa IU/mg, calculated on the dried basis. The ratio of Anti-Factor Xa activity to Anti-Factor IIa activity is between 3.3 and 5.3.
IDENTIFICATION
• A. Ultraviolet Absorption  197U
197U
Sample solution:
500 µg/mL
Medium:
0.01 N hydrochloric acid. The spectra exhibit maxima at 231 ± 2 nm.
• B. 13C NMR Spectrum
Standard solution:
Dissolve 200 mg of USP Enoxaparin Sodium RS in a mixture of 0.2 mL of deuterium oxide and 0.8 mL of water. Add 0.05 mL of deuterated methanol to serve as an internal reference.
Sample solution:
Dissolve 200 mg of Enoxaparin Sodium in a mixture of 0.2 mL of deuterium oxide and 0.8 mL of water. Add 0.05 mL of deuterated methanol.
Analysis:
Transfer the Standard solution and the Sample solution to NMR tubes of 5-mm diameter. Using a pulsed (Fourier transform) NMR spectrometer operating at NLT 75 MHz for 13C, record the 13C NMR spectra of the Standard solution and the Sample solution at 40 .
.
Acceptance criteria:
The spectra are similar.
• C.
The ratio of the numerical value of the Anti-Factor Xa activity, in Anti-Factor Xa IU/mg, to the numerical value of the Anti-Factor IIa activity, in Anti-Factor IIa IU/mg, as determined by the Assay (Anti-Factor Xa Activity) and Anti-Factor IIa Activity, respectively, is NLT 3.3 and NMT 5.3.
• D. Molecular Weight Distribution and Weight-Average Molecular Weight
Mobile phase:
Prepare a 0.5 M lithium nitrate solution. Pass through a membrane filter of 0.45-µm or smaller pore size, and degas with helium.
Standard solution:
10 mg/mL of USP Enoxaparin Sodium RS in Mobile phase
Sample solution:
10 mg/mL of Enoxaparin Sodium in Mobile phase
Chromatographic system
Mode:
Size exclusion LC
Detector:
Differential refractive index
Column
Guard:
6-mm × 40-mm; packing L59
Analytical:
Two 7.8-mm × 300-mm columns in series; packing L59
Temperature:
Room temperature
Flow rate:
0.6 mL/min maintained constant to ± 1.0%
Analysis:
Reconstitute 1 vial each of USP Enoxaparin Sodium Molecular Weight Calibrant A RS and USP Enoxaparin Sodium Molecular Weight Calibrant B RS in 1 mL of Mobile phase. Separately inject 20 µL of USP Enoxaparin Sodium Molecular Weight Calibrant A RS and USP Enoxaparin Sodium Molecular Weight Calibrant B RS, record the chromatograms, and measure the retention times. Inject in duplicate 20 µL each of the Standard solution and the Sample solution, and record the chromatograms for a length of time to ensure complete elution, including salt and solvent peaks. Calculate the total peak areas under each of the Standard solution and Sample solution chromatograms, excluding salt and solvent peaks at the end.
Calibration curve:
Plot the retention times on the x-axis against the peak molecular weights on the y-axis for the peaks in the chromatograms of USP Enoxaparin Sodium Molecular Weight Calibrant A RS and USP Enoxaparin Sodium Molecular Weight Calibrant B RS, and fit the data to a third-order polynomial using suitable gel permeation chromatography (GPC) software.
Calculations:
Compute the data, using the same GPC software, and determine the weight-average molecular weight, Mw, for each of the duplicate chromatograms of the Standard solution and the Sample solution, and take the average for each solution. Correct the mean value of Mw to the nearest 50. The Chromatographic system is suitable if Mw of USP Enoxaparin Sodium RS is within 150 Da of the labeled Mw value. The Mw for the Sample solution is between 3800 and 5000 Da. Using the same software, determine for each of the duplicate Sample solution chromatograms the percentage of Enoxaparin Sodium chains with molecular weights lower than 2000 Da, M2000, the percentage of Enoxaparin Sodium chains with molecular weights in the range 2000–8000 Da, M2000–8000, and the percentage of Enoxaparin Sodium chains with molecular weights greater than 8000 Da, M8000. Average the duplicate values and express to the nearest 0.5%.
Acceptance criteria:
M2000 is between 12.0% and 20.0%, M2000–8000 is between 68.0% and 82.0%, and M8000 is NMT 18.0%.
• E. Identification Tests—General, Sodium  191
191 :
Meets the requirements
:
Meets the requirements
ASSAY
• Anti-Factor Xa Activity
Acetic acid solution:
Glacial acetic acid and water (42:58)
pH 7.4 polyethylene glycol 6000 buffer:
Dissolve 6.08 g of tris(hydroxymethyl)aminomethane and 8.77 g of sodium chloride in 500 mL of water. Add 1.0 g of polyethylene glycol 6000, adjust with hydrochloric acid to a pH of 7.4, and dilute with water to 1000 mL.
pH 7.4 buffer:
Dissolve 6.08 g of tris(hydroxymethyl)aminomethane and 8.77 g of sodium chloride in 500 mL of water. Adjust with hydrochloric acid to a pH of 7.4, and dilute with water to 1000 mL.
pH 8.4 buffer:
Dissolve 3.03 g of tris(hydroxymethyl)aminomethane, 5.12 g of sodium chloride, and 1.40 g of edetate sodium in 250 mL of water. Adjust with hydrochloric acid to a pH of 8.4, and dilute with water to 500 mL.
Human antithrombin III solution:
Reconstitute a vial of antithrombin III (see Reagents, Indicators, and Solutions—Reagent Specifications) in water to obtain a solution containing 5 Antithrombin III Units/mL. Dilute this solution with pH 7.4 polyethylene glycol 6000 buffer to obtain a solution having a concentration of 1.0 Antithrombin III Unit/mL.
Factor Xa solution:
Reconstitute a weighed quantity of bovine factor Xa (see Reagents, Indicators, and Solutions—Reagent Specifications) in pH 7.4 polyethylene glycol 6000 buffer to obtain a solution that gives an increase in absorbance value at 405 nm of NMT 0.20 absorbance units/min when assayed as described below but using as an appropriate volume, V, the volume in µL of pH 7.4 buffer instead of V µL of the enoxaparin solution.
Chromogenic substrate solution:
Prepare a solution of a suitable chromogenic substrate for amidolytic test (see Reagents, Indicators, and Solutions—Reagent Specifications) for factor Xa in water to obtain a concentration of about 3 mM. Dilute with pH 8.4 buffer to obtain a solution having a concentration of 0.5 mM.
Standard solutions:
Dilute USP Enoxaparin Sodium Solution for Bioassays RS with pH 7.4 buffer to obtain four dilutions in the concentration range between 0.025 and 0.2 Anti-Factor Xa IU/mL.
Sample solutions:
Proceed as directed for Standard solutions to obtain concentrations of Enoxaparin Sodium similar to those obtained for the Standard solutions.
Analysis
Samples:
Standard solutions, Sample solutions, Human antithrombin III solution, pH 7.4 buffer, Factor Xa solution, Chromogenic substrate solution, and Acetic acid solution
Label 18 suitable tubes: B1 and B2 for blanks; T1, T2, T3, and T4 each in duplicate for the dilutions of the Sample solutions; and S1, S2, S3, and S4 each in duplicate for the dilutions of the Standard solutions. [Note—Treat the tubes in the order B1, S1, S2, S3, S4, T1, T2, T3, T4, T1, T2, T3, T4, S1, S2, S3, S4, B2. ] To each tube add the same volume, V (20–50 µL), of Human antithrombin III solution and an equal volume, V, of either the blank (pH 7.4 buffer) or an appropriate dilution of the Sample solutions or the Standard solutions. Mix, but do not allow bubbles to form. Incubate at 37 for 1.0 min. Add to each tube 2V (40–100 µL) of Factor Xa solution, and incubate for 1.0 min. Add 5V (100–250 µL) volume of Chromogenic substrate solution. Stop the reaction after 4.0 min with 5V (100–250 µL) volume of Acetic acid solution. Measure the absorbance of each solution at 405 nm using a suitable spectrometer (see Spectrophotometry and Light-Scattering
for 1.0 min. Add to each tube 2V (40–100 µL) of Factor Xa solution, and incubate for 1.0 min. Add 5V (100–250 µL) volume of Chromogenic substrate solution. Stop the reaction after 4.0 min with 5V (100–250 µL) volume of Acetic acid solution. Measure the absorbance of each solution at 405 nm using a suitable spectrometer (see Spectrophotometry and Light-Scattering  851
851 ) against blank B1. The reading of blank B2 relative to the blank B1 is NMT ± 0.05 absorbance unit.
) against blank B1. The reading of blank B2 relative to the blank B1 is NMT ± 0.05 absorbance unit.
Calculations:
For each series, calculate the regression of the absorbance against log concentrations of the Sample solutions and of the Standard solutions, and calculate the potency of the Enoxaparin Sodium in IU of Anti-Factor Xa activity/mL using statistical methods for parallel-line assays. The four independent log relative potency estimates are then combined to obtain the final geometric mean. Its confidence limits are calculated. Express the Anti-Factor Xa activity of Enoxaparin Sodium/mg.
Acceptance criteria:
The potency is NLT 90 and NMT 125 Anti-Factor Xa IU/mg on the dried basis.
OTHER COMPONENTS
• Benzyl Alcohol Content
Mobile phase:
Acetonitrile, methanol, and water (3:1:16)
Standard solution:
0.1 mg/mL of USP Benzyl Alcohol RS in water
Sample solution:
Weigh 0.5 g of Enoxaparin Sodium into a 10-mL volumetric flask, and dissolve in 5.0 mL of 1 N sodium hydroxide. Allow to stand at room temperature for about 1 h. Add 1.0 mL of glacial acetic acid, dilute with water to volume, and mix.
Chromatographic system
Mode:
LC
Detector:
UV 256 nm
Column:
4.6-mm × 15-cm stainless steel; packing L7
Flow rate:
1.0 mL/min, maintained constant to ±10%
Injection size:
20 µL
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of benzyl alcohol in the portion of Enoxaparin Sodium taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak area of benzyl alcohol from the Sample solution |
| rS | = | = peak area of benzyl alcohol from the Standard solution |
| CS | = | = concentration of benzyl alcohol in the Standard solution (mg/mL) |
| CU | = | = concentration of Enoxaparin Sodium in the Sample solution (mg/mL) |
Acceptance criteria:
NMT 0.1%
• Nitrogen Determination, Method II  461
461 :
1.8%–2.5%, on the dried basis
:
1.8%–2.5%, on the dried basis
• Sodium Content
Cesium chloride solution:
1.27 mg/mL of cesium chloride in 0.1 N hydrochloric acid
Standard solution A:
0.0025% of sodium chloride in Cesium chloride solution
Standard solution B:
0.0050% of sodium chloride in Cesium chloride solution
Standard solution C:
0.0075% of sodium chloride in Cesium chloride solution
Sample solution:
Transfer 50.0 mg of Enoxaparin Sodium to a 100-mL volumetric flask, and dissolve in and dilute with Cesium chloride solution to volume.
Analysis
Samples:
Standard solution A, Standard solution B, Standard solution C, Cesium chloride solution, and Sample solution
Concomitantly determine the absorbances of the Cesium chloride solution (blank), Sample solution, and Standard solutions at 330.3 nm using a sodium hollow-cathode lamp and an air–acetylene flame. Using the absorbances of Standard solutions A–C, determine the sodium content in the Sample solution after appropriate blank correction.
Acceptance criteria:
11.3%–13.5% on the dried basis
IMPURITIES
Inorganic Impurities
• Heavy Metals, Method I  231
231 :
NMT 30 ppm using a 5% solution in water.
:
NMT 30 ppm using a 5% solution in water.
SPECIFIC TESTS
• pH  791
791 :
6.2–7.7 for a 10.0% solution in water
:
6.2–7.7 for a 10.0% solution in water
• Loss on Drying  731
731 :
Dry 1 g in a vacuum at 70
:
Dry 1 g in a vacuum at 70 for 6 h: it loses NMT 10.0% of its weight.
for 6 h: it loses NMT 10.0% of its weight.
• Specific Absorbance
Sample solution:
0.5 mg/mL of Enoxaparin Sodium in 0.01 N hydrochloric acid
Analysis:
Obtain the UV spectra of the Standard solution and the Sample solution between 200 nm and 300 nm against 0.01 N hydrochloric acid blank. Calculate the specific absorbance at the wavelength of maximum absorbance at 231 ± 2 nm, with reference to the dried substance:
Result = A × 100 × 1000/[M × l × (100  E)]
E)]
| A | = | = absorbance at the wavelength of maximum absorbance |
| M | = | = weight of Enoxaparin Sodium in the Sample solution (mg) |
| l | = | = pathlength (typically l = 1 cm) |
| E | = | = loss on drying (%) |
Acceptance criteria:
14.0–20.0, on the dried basis
• Bacterial Endotoxins Test  85
85 :
It contains NMT 0.01 USP Endotoxin Unit/IU of Anti-Factor Xa activity.
:
It contains NMT 0.01 USP Endotoxin Unit/IU of Anti-Factor Xa activity.
• Anti-Factor IIa Activity
Acetic acid solution, pH 7.4 polyethylene glycol 6000 buffer, pH 7.4 buffer, pH 8.4 buffer, and Human antithrombin III solution:
Proceed as directed in the Assay for Anti-Factor Xa Activity, except that the concentration of the Human antithrombin III solution is 0.5 Antithrombin III Unit/mL.
Thrombin human solution:
Reconstitute thrombin human (see Reagents, Indicators and Solutions—Reagent Specifications) in water, and dilute in pH 7.4 polyethylene glycol 6000 buffer to obtain a solution having a concentration of 5 Thrombin Units/mL.
Chromogenic substrate solution:
Prepare a solution of a suitable chromogenic substrate for an amidolytic test (see Reagents, Indicators, and Solutions—Reagent Specifications) for thrombin in water to obtain a concentration of about 3 mM. Immediately before use, dilute with pH 8.4 buffer to 0.5 mM.
Standard solutions:
Dilute USP Enoxaparin Sodium Solution for Bioassays RS with pH 7.4 buffer to obtain four dilutions having concentrations in the range between 0.015 and 0.075 IU of Anti-Factor IIa activity/mL.
Sample solutions:
Proceed as directed under Standard solutions to obtain concentrations of Enoxaparin Sodium similar to those obtained for the Standard solutions.
Analysis:
Proceed as directed in the Assay for Anti-Factor Xa Activity, except to use Thrombin human solution instead of Factor Xa solution and to use the Human antithrombin III solution as described above.
Calculations:
For each series, calculate the regression of the absorbance against log concentrations of the Sample solutions and of the Standard solutions, and calculate the potency of the Enoxaparin Sodium in IU of Anti-Factor IIa activity/mg using statistical methods for parallel-line assays. The four independent dilution estimates are then combined to obtain the final weighted mean. Then calculate the confidence limits. Express the Anti-Factor IIa activity of Enoxaparin Sodium/mg.
Acceptance criteria:
It has a potency of NLT 20.0 and NMT 35.0 Anti-Factor IIa IU/mg on the dried basis.
• Molar Ratio of Sulfate to Carboxylate
Mobile phase:
Carbon dioxide-free water
Sample solution:
5 mg/mL of Enoxaparin Sodium in carbon dioxide-free water
Chromatographic system
Mode:
LC
Detector:
Ion
Column:
Two columns; one 1.5-cm × 2.5-cm column packed with an anion-exchange resin L64 packing and one 1.5-cm × 7.5-cm column packed with a cation-exchange resin L65 packing. The outlet of the anion-exchange column is connected to the inlet of the cation-exchange column.
Flow rate:
1 mL/min
Analysis
Sample:
Sample solution
[Note—Regenerate the anion-exchange column and the cation-exchange column with 1 N sodium hydroxide and 1 N hydrochloric acid, respectively, between two injections. ]
Inject the Sample solution into the anion-exchange column, and collect the eluate from the cation-exchange column in a beaker at the outlet until the ion detector reading returns to the baseline value. Quantitatively transfer the eluate to a titration vessel containing a magnetic stirring bar, and dilute with carbon dioxide-free water to about 60 mL. Position the titration vessel on a magnetic stirrer, and immerse the electrodes. Note the initial conductivity reading, and titrate with approximately 0.1 N sodium hydroxide added in 100-µL portions. [Note—Prepare the sodium hydroxide solution in carbon dioxide-free water. ] Record the buret reading and the conductivity meter reading after each addition of the sodium hydroxide solution.
Calculations:
Plot the conductivity measurements on the y-axis against the volumes of sodium hydroxide added on the x-axis. The graph will have three linear sections—an initial downwards slope, a middle slight rise, and a final rise. For each of these sections draw the best-fit straight lines using linear regression analysis. At the points where the first and second straight lines intersect and where the second and third lines intersect, draw perpendiculars to the x-axis to determine the volumes of sodium hydroxide taken up by the sample at those points. The point where the first and second lines intersect corresponds to the volume of sodium hydroxide taken up by the sulfate groups (VS). The point where the second and third lines intersect corresponds to the volume of sodium hydroxide consumed by the sulfate and the carboxylate groups together (VT). Calculate the molar ratio of sulfate to carboxylate:
Result = VS/(VT  VS)
VS)
Acceptance criteria:
The molar ratio of sulfate to carboxylate is NLT 1.8.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers, and store below 40 , preferably at room temperature.
, preferably at room temperature.
• USP Reference Standards  11
11
USP Endotoxin RS
USP Enoxaparin Sodium Molecular Weight Calibrant A RS
USP Enoxaparin Sodium Molecular Weight Calibrant B RS
USP Enoxaparin Sodium Solution for Bioassays RS
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Anita Y. Szajek, Ph.D.
Principal Scientific Liaison 1-301-816-8325 |
(BIO12010) Monographs - Biologics and Biotechnology 1 |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3040
Pharmacopeial Forum: Volume No. 37(1)