Efavirenz Capsules
DEFINITION
Efavirenz Capsules contain NLT 92.0% and NMT 108.0% of the labeled amount of efavirenz (C14H9ClF3NO2).
IDENTIFICATION
• A. Infrared Absorption  197K
197K
Sample solution:
Dissolve the contents of 1 Capsule in about 5 mL of acetonitrile by mixing on a vortex mixer. Allow to settle, remove about 3 mL of the solution, and centrifuge for about 5 min. Transfer 1–2 mL of supernatant to a clean suitable container, and evaporate to dryness under nitrogen. Mix 0.5–1 mg of the powder with 200 mg of potassium bromide.
• B. Ultraviolet Absorption  197U
197U
Solvent:
Acetonitrile
Standard solution:
10 µg/mL in Solvent
Sample solution:
Dissolve the contents of 1 Capsule in about 40 mL of Solvent by shaking for about 30 min. Pass through a suitable nylon or PVDF membrane filter, discarding the first 2 mL of filtrate, and dilute a portion with acetonitrile to a concentration of 10 µg/mL of efavirenz.
Acceptance criteria:
The UV absorption spectrum of the Sample solution exhibits maxima and minima at the same wavelengths as does the Standard solution.
ASSAY
• Procedure
Diluent:
Acetonitrile and water (1:1)
Solution A:
Methanol, trifluoroacetic acid, and water (1:0.005:9). [Note—Use only freshly-opened trifluoroacetic acid, 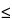 6 months. ]
6 months. ]
Solution B:
Methanol, trifluoroacetic acid, and water (9:0.005:1). [Note—Use only freshly-opened trifluoroacetic acid, 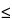 6 months. ]
6 months. ]
Mobile phase:
See the gradient table below.
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 60 | 40 |
| 16 | 50 | 50 |
| 23 | 35 | 65 |
| 28 | 30 | 70 |
| 29 | 20 | 80 |
| 31 | 20 | 80 |
| 32 | 60 | 40 |
| 40 | 60 | 40 |
Standard solution 1:
0.2 mg/mL of USP Efavirenz Related Compound B RS in Diluent
Standard solution 2:
5 mg/mL of USP Efavirenz RS in acetonitrile. [Note—Sonicate to dissolve before diluting to final volume. ]
Standard solution:
250 µg/mL of USP Efavirenz RS and 1 µg/mL of USP Efavirenz Related Compound B RS in Diluent prepared from Standard solution 2 and Standard solution 1, respectively. [Note—Store protected from light. For the HPLC analysis, it is recommended to use polypropylene vials, because degradation has been noted with certain brands made of glass. ]
Sample stock solution:
Transfer the contents of NLT 10 Capsules to a suitable container, and extract the contents in acetonitrile by mixing for about 30 min to obtain a quantitative solution equivalent to about 5 mg/mL of efavirenz. [Note—Store protected from light. ]
Sample solution:
Filter a portion of the Sample stock solution, and dilute the filtrate with Diluent to obtain a solution of about 250 µg/mL of efavirenz. [Note—Store protected from light. For the HPLC analysis, it is recommended to use polypropylene vials, because degradation has been noted with certain brands made of glass. ]
Chromatographic system
Mode:
LC
Detector:
UV 250 nm
Column:
4.6-mm × 15-cm; packing L10
Column temperature:
40
Flow rate:
1.5 mL/min
Injection size:
35 µL
System suitability
Sample:
Standard solution
Suitability requirements
Resolution:
NLT 1.2 between efavirenz related compound B and efavirenz
Relative standard deviation:
NMT 2.0% for efavirenz
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of efavirenz (C14H9ClF3NO2) in the portion of Capsules taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of efavirenz from the Sample solution |
| rS | = | = peak response of efavirenz from the Standard solution |
| CS | = | = concentration of USP Efavirenz RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of efavirenz in the Sample solution (mg/mL) |
Acceptance criteria:
92.0%–108.0%
PERFORMANCE TESTS
• Dissolution  711
711
Medium:
1.0% (w/v) sodium lauryl sulfate in water; 900 mL. [Note—Do not deaerate. ]
Apparatus 2:
50 rpm, with helix sinker
Time:
45 min
Standard solution:
(L/900) mg/mL of USP Efavirenz RS in Medium, where L is the Capsule label claim in mg. A small volume of methanol, NMT 10% of the final volume, could be used to solubilize efavirenz. Dilute this solution with Medium to obtain a final concentration of about 0.01 mg/mL for Capsules labeled to contain 50 mg, or about 0.02 mg/mL for Capsules labeled to contain 100 mg or 200 mg.
Sample solution:
Pass a portion of the solution under test through a suitable filter of 0.45-µm pore size, and dilute with Medium to obtain a theoretical concentration similar to the Standard solution, assuming complete dissolution of the Capsule label claim.
Analytical wavelength:
UV 247 nm
Cell:
1 cm
Blank:
Medium
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of efavirenz dissolved:
Result = (AU/AS) × (CS/L) × D × V × 100
| AU | = | = absorbance of the Sample solution |
| AS | = | = absorbance of the Standard solution |
| CS | = | = concentration of the Standard solution (mg/mL) |
| L | = | = label claim (mg/Capsule) |
| D | = | = dilution factor of the Sample solution |
| V | = | = volume of Medium, 900 mL |
Tolerances:
NLT 80% (Q) of the labeled amount of efavirenz is dissolved.
• Uniformity of Dosage Units  905
905 :
Meet the requirements
:
Meet the requirements
Procedure for content uniformity
Standard solution:
10 µg/mL of USP Efavirenz RS in acetonitrile
Sample solution:
Transfer the contents of 1 Capsule into a suitable container, and dissolve in 40.0 mL of acetonitrile. Shake for about 30 min and pass through a suitable nylon or PVDF membrane filter. Dilute a portion of the filtrate to an efavirenz concentration of about 10 µg/mL.
Spectrometric conditions
Mode:
UV absorption spectroscopy
Analytical wavelength:
UV 246 nm
Cell:
1 cm
Blank:
Acetonitrile
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of efavirenz (C14H9ClF3NO2) in the portion of Capsules taken:
Result = (AU/AS) × (CS/L) × V × D × 100
| AU | = | = absorbance of efavirenz from the Sample solution |
| AS | = | = absorbance of efavirenz from the Standard solution |
| CS | = | = concentration of USP Efavirenz RS in the Standard solution (mg/mL) |
| L | = | = label claim (mg/Capsule) |
| V | = | = volume of the Sample solution |
| D | = | = dilution factor of the Sample solution |
IMPURITIES
Organic Impurities
• Procedure
Diluent, Solution A, Solution B, Sample solution, and Chromatographic system:
Prepare as directed in the Assay.
System suitability solution:
Use the Standard solution prepared as directed in the Assay.
Standard solution:
1.25 µg/mL of USP Efavirenz RS and 0.005 µg/mL of USP Efavirenz Related Compound B RS in Diluent from the System suitability solution
System suitability
Samples:
System suitability solution and Standard solution
Suitability requirements
Resolution:
NLT 1.2 between efavirenz related compound B and efavirenz, System suitability solution
Relative standard deviation:
NMT 5.0% for efavirenz, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of any individual impurity in the portion of Capsules taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
| rU | = | = peak response of any individual impurity (degradation product) from the Sample solution |
| rS | = | = peak response of efavirenz from the Standard solution |
| CS | = | = concentration of USP Efavirenz RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of efavirenz in the Sample solution (mg/mL) |
| F | = | = relative response factor (see Impurity Table 1) |
Acceptance criteria
Individual impurities:
See Impurity Table 1. [Note—Disregard any peak less than 0.05%. ]
Total impurities:
NMT 0.50%. [Note—Include only the degradation products in the calculation of the total impurities. ]
Impurity Table 1
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Efavirenz aminoalcohol (degradation product)a | 0.48 | 0.26 | 0.25 |
| Efavirenz ethene analogb | 0.93 | — | * |
| Efavirenz pent-3-ene-1-yne (cis)c | 1.16 | — | * |
| Efavirenz pent-3-ene-1-yne (trans)d | 1.16 | — | * |
| Efavirenz penteneynee | 1.16 | — | * |
| Efavirenz pentyne analogf | 1.2 | — | * |
| Methylefavirenzg | 1.28 | — | * |
| Efavirenz aminoalcohol methyl carbamateh | 1.33 | — | * |
| N-Benzylefavirenzi | 1.8 | — | * |
| Efavirenz benzoylaminoalcoholj | 1.9 | — | * |
| Quinoline analog (degradation product)k | 1.45 | 2.0 | 0.20 |
| Efavirenz aminoalcohol ethyl carbamatel | 1.53 | — | * |
| Unidentified impurity | 1.60 | — | * |
| Efavirenz aminoalcohol bis(ethoxycarbonyl)m | 1.63 | — | * |
| Unidentified impurity | 2.1 | — | * |
| Cyclobutenylindole analogn | 2.18 | — | * |
| Any other individual degradation product | — | 1.0 | 0.20 |
|
a
(S)-2-(2-Amino-5-chlorophenyl)-4-cyclopropyl-1,1,1-trifluorobut-3-yn-2-ol.
b
(S,E)-6-Chloro-4-(2-cyclopropylvinyl)-4-(trifluoromethyl)-2H-3,1-benzoxazin-2-one.
c
(S,E)-6-Chloro-4-(pent-3-en-1-ynyl)-4-(trifluoromethyl)-2H-3,1-benzoxazin-2-one.
d
(S,Z)-6-Chloro-4-(pent-3-en-1-ynyl)-4-(trifluoromethyl)-2H-3,1-benzoxazin-2-one.
e
(S)-6-Chloro-4-(3-methylbut-3-en-1-ynyl)-4-(trifluoromethyl)-2H-3,1-benzoxazin-2-one.
f
(S)-6-Chloro-4-(pent-1-ynyl)-4-(trifluoromethyl)-2H-3,1-benzoxazin-2-one.
g
(S)-6-Chloro-4-{[(2RS,2RS)-2-methylcyclopropyl]ethynyl}-4-(trifluoromethyl)-2H-3,1-benzoxazin-2-one.
h
(S)-Methyl 4-chloro-2-(4-cyclopropyl-1,1,1-trifluoro-2-hydroxybut-3-yn-2-yl)phenylcarbamate.
i
(S)-6-Chloro-4-(cyclopropylethynyl)-1-(4-methoxybenzyl)-4-(trifluoromethyl)-2H-3,1-benzoxazin-2-one.
j
(S)-N-[4-Chloro-2-(4-cyclopropyl-1,1,1-trifluoro-2-hydroxybut-3-yn-2-yl)phenyl]-4-methoxybenzamide.
k
6-Chloro-2-cyclopropyl-4-(trifluoromethyl)quinoline.
l
(S)-Ethyl 4-chloro-2-(4-cyclopropyl-1,1,1-trifluoro-2-hydroxybut-3-yn-2-yl)phenylcarbamate.
m
(S)-Ethyl 4-chloro-2-[4-cyclopropyl-2-(ethoxycarbonyloxy)-1,1,1-trifluorobut-3-yn-2-yl]phenylcarbamate.
n
Ethyl 5-chloro-2-cyclobutenyl-3-(trifluoromethyl)-1H-indole-1-carboxylate.
*
For information purposes only. These are process impurities monitored in the drug substance and are not included in the total impurities.
|
|||
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Store in well-closed containers at controlled room temperature.
• USP Reference Standards  11
11
USP Efavirenz Related Compound B RS
(S,E)-6-Chloro-4-(2-cyclopropylvinyl)-4-(trifluoromethyl)-2H-3,1-benzoxazin-2-one.
C14H11ClF3NO2 317.69
(S,E)-6-Chloro-4-(2-cyclopropylvinyl)-4-(trifluoromethyl)-2H-3,1-benzoxazin-2-one.
C14H11ClF3NO2 317.69
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Behnam Davani, Ph.D., M.B.A.
Senior Scientific Liaison 1-301-816-8394 |
(SM12010) Monographs - Small Molecules 1 |
| Margareth R.C. Marques, Ph.D.
Senior Scientific Liaison 1-301-816-8106 |
(GCDF2010) General Chapters - Dosage Forms | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3013
Pharmacopeial Forum: Volume No. 36(3) Page 664
