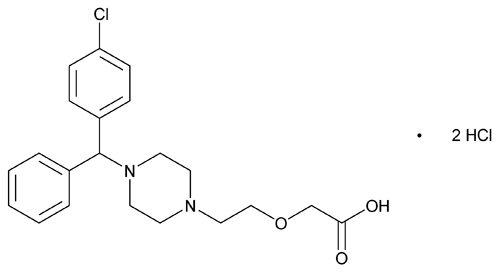
Cetirizine Hydrochloride
C21H25ClN2O3·2HCl 461.81
(±)-[2-[4-[(4-Chlorophenyl)phenylmethyl]-1-piperazinyl]ethoxy]acetic acid, dihydrochloride;
(±)-[2-[4-(p-Chloro- -phenylbenzyl)-1-piperazinyl]ethoxy]acetic acid, dihydrochloride
-phenylbenzyl)-1-piperazinyl]ethoxy]acetic acid, dihydrochloride


 [83881-52-1].
[83881-52-1].
(se tir' i zeen hye'' droe klor' ide).
C21H25ClN2O3·2HCl 461.81
(±)-[2-[4-[(4-Chlorophenyl)phenylmethyl]-1-piperazinyl]ethoxy]acetic acid, dihydrochloride;
(±)-[2-[4-(p-Chloro-
DEFINITION
Cetirizine Hydrochloride contains NLT 98.0% and NMT 102.0% of C21H25ClN2O3·2HCl, calculated on the dried basis.
IDENTIFICATION
• B. Identification Test—General, Chloride  191
191 :
Meets the requirements
:
Meets the requirements
ASSAY
• Procedure
Mobile phase:
Acetonitrile, water, and 1 M sulfuric acid (93:6.6:0.4)
Standard solution:
0.5 mg/mL USP Cetirizine Hydrochloride RS in Mobile phase
Sample solution:
0.5 mg/mL Cetirizine Hydrochloride in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 230 nm
Column:
4.6-mm × 25-cm; 5-µm packing L3
Flow rate:
1 mL/min
Injection size:
10 µL
System suitability
Sample:
Standard solution
Suitability requirements
Tailing factor:
NMT 2.0
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C21H25ClN2O3·2HCl in the portion of Cetirizine Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Cetirizine Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Cetirizine Hydrochloride in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% on the dried basis
IMPURITIES
Organic Impurities
[Note—It is recommended that Test 2 be performed if either cetirizine ethanol (2-{4-[(4-chlorophenyl)phenylmethyl]piperazin-1-yl}ethanol) or cetirizine acetic acid (2-{4-[(4-chlorophenyl)phenylmethyl]piperazin-1-yl}acetic acid) may be present in the test substance. ]
• Procedure 1
Mobile phase and Sample solution:
Proceed as directed in the Assay.
System suitability solution:
4 µg/mL each of USP Cetirizine Hydrochloride RS and USP Cetirizine Related Compound A RS in Mobile phase
Standard solution:
0.5 µg/mL of USP Cetirizine Hydrochloride RS in Mobile phase
Chromatographic system:
Prepare as directed in the Assay.
Run time:
Three times the retention time of cetirizine
System suitability
Samples:
Standard solution and System suitability solution
Suitability requirements
Tailing factor:
NMT 2.0 for cetirizine, System suitability solution
Resolution:
NLT 2.0 between cetirizine and cetirizine related compound A, System suitability solution
Relative standard deviation:
NMT 2.0% cetirizine, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Cetirizine hydrochloride taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
| rU | = | = peak response for each impurity from the Sample solution |
| rS | = | = peak response for cetirizine from the Standard solution |
| CS | = | = concentration of USP Cetirizine Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Cetirizine Hydrochloride in the Sample solution (mg/mL) |
| F | = | = relative response factor (see Impurity Table 1 for values) |
Acceptance criteria:
See Impurity Table 1.
Total impurities:
NMT 0.3%. [Note—Disregard peaks below 0.02%. ]
Impurity Table 1
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| 4-CBHa | 0.3 | 1.4 | 0.1 |
| Dimerb | 0.5 | 1.8 | 0.1 |
| 2-Chlorocetirizinec | 0.85 | 0.49 | 0.1 |
| Cetirizine related compound Ad | 0.9 | 0.95 | 0.1 |
| Cetirizine | 1.0 | — | — |
| Deschlorocetirizinee | 1.4 | 0.45 | 0.1 |
| CBHPf | 1.45 | 1.6 | 0.1 |
| Any individual unspecified impurity | — | 1.0 | 0.1 |
|
a
4-Chlorobenzhydrol.
b
1,4-Bis[(4-chlorophenyl)phenylmethyl]piperazine.
c
2-[2-[4-[(2-Chlorophenyl)phenylmethyl]piperazin-1-yl]ethoxy]acetic acid.
d
2-[2-[4-[(4-Chlorophenyl)phenylmethyl]piperazin-1-yl]ethoxy]acetic acid, ethyl ester (cetirizine ethyl ester).
e
2-[2-[4-(Diphenylmethyl]piperazin-1-yl]ethoxy]acetic acid.
f
1-[(4-Chlorophenyl)phenylmethyl]piperazine.
|
|||
• Procedure 2
Solution A:
2 g/L tetrabutyl ammonium hydrogen sulfate and 3 g/L of monobasic sodium phosphate monohydrate in water. Adjust with 1 N sodium hydroxide to a pH of 2.8 ± 0.05.
Solution B:
Methanol
Buffer:
1.4 g/L monobasic sodium phosphate monohydrate and 2.7 g/L of dibasic sodium phosphate heptahydrate. Adjust with either 1 N sodium hydroxide or 10% phosphoric acid to a pH of 6.9 ± 0.1.
Diluent:
Acetonitrile and Buffer (1:1)
Mobile phase:
See the gradient table below.
| Time (min) |
Solution A (%) |
Solution B (%) |
Flow Rate (mL/min) |
|---|---|---|---|
| 0 | 58 | 42 | 1.2 |
| 40 | 58 | 42 | 1.2 |
| 68 | 20 | 80 | 1.5 |
| 108 | 20 | 80 | 1.5 |
| 110 | 58 | 42 | 1.2 |
| 120 | 58 | 42 | 1.2 |
Standard solution:
2 µg/mL of USP Cetirizine Hydrochloride RS in Diluent
Sample solution:
2 mg/mL cetirizine hydrochloride in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 232 nm
Column:
4.6-mm × 25-cm; 5-µm packing L1
Column temperature:
40
Injection size:
10 µL
System suitability
Sample:
Standard solution
Suitability requirements
Tailing factor:
NMT 2
Column efficiency:
NLT 6000 theoretical plates
Relative standard deviation:
NMT 5.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Cetirizine Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
| rU | = | = peak response for each impurity from the Sample solution |
| rS | = | = peak response for cetirizine from the Standard solution |
| CS | = | = concentration of USP Cetirizine Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Cetirizine Hydrochloride in the Sample solution (mg/mL) |
| F | = | = relative response factor (see Impurity Table 2 for values) |
Acceptance criteria:
See Impurity Table 2.
Total impurities:
NMT 0.3%. [Note—Disregard peaks below 0.05%. ]
Impurity Table 2
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Deschlorocetirizinea | 0.35 | 0.56 | 0.1 |
| Cetirizine ethanolb | 0.53 | 1.2 | 0.1 |
| CBHPc | 0.66 | 1.3 | 0.1 |
| 2-Chlorocetirizined | 0.70 | 0.52 | 0.1 |
| Cetirizine methyl estere | 0.81 | 0.96 | 0.1 |
| 3-Chlorocetirizinef | 0.87 | 0.52 | 0.1 |
| Cetirizine | 1.0 | — | — |
| Cetirizine acetic acidg | 1.15 | 0.97 | 0.1 |
| Cetirizine N-oxideh | 1.25 | 0.81 | 0.1 |
| 4-CBHi | 1.55 | 1.2 | 0.1 |
| 4-Chlorobenzophenonej | 1.66 | 0.50 | 0.1 |
| Cetirizine dimerk | 2.48 | 1.4 | 0.1 |
| Any individual unspecified impurity | — | 1.0 | 0.10 |
|
a
2-{2-[4-(Diphenylmethyl)piperazin-1-yl]ethoxy}acetic acid.
b
2-{4-[(4-Chlorophenyl)phenylmethyl]piperazin-1-yl}ethanol.
c
1-[(4-Chlorophenyl)phenylmethyl]piperazine.
d
2-(2-{4-[(2-Chlorophenyl)phenylmethyl]piperazin-1-yl}ethoxy)acetic acid.
e
Methyl 2-(2-{4-[(4-chlorophenyl)phenylmethyl]piperazin-1-yl}ethoxy)acetate.
f
2-[2-[4-[(3-Chlorophenyl)phenylmethyl]piperazin-1-yl]ethoxy]acetic acid.
g
2-{4-[(4-Chlorophenyl)phenylmethyl]piperazin-1-yl}acetic acid.
h
2-(2-{4-[(4-Chlorophenyl)(phenyl)methyl]piperazin-1-yl}ethoxy)acetic acid N1-oxide.
i
4-Chlorobenzhydrol.
j
(4-Chlorophenyl)phenylmethanone.
k
1,4-Bis[(4-chlorophenyl)phenylmethyl]piperazine.
|
|||
SPECIFIC TESTS
• pH  791
791 :
1.2-1.8, in an aqueous solution 1 in 20
:
1.2-1.8, in an aqueous solution 1 in 20
• Loss on Drying  731
731 :
Dry a sample at 105
:
Dry a sample at 105  to a constant weight: it loses NMT 0.5% of its weight.
to a constant weight: it loses NMT 0.5% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers, protected from light and moisture. Store at room temperature.
• Labeling:
Label it to indicate with which impurity procedures the article complies.
• USP Reference Standards  11
11
USP Cetirizine Related Compound A RS
(RS)-2-[2-[4-[(4-Chlorophenyl)phenylmethyl]piperazin-1-yl]ethoxy]acetic acid ethyl ester dihyrochloride.
C23H29ClN2O3·2HCl 489.86
(RS)-2-[2-[4-[(4-Chlorophenyl)phenylmethyl]piperazin-1-yl]ethoxy]acetic acid ethyl ester dihyrochloride.
C23H29ClN2O3·2HCl 489.86
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Domenick Vicchio, Ph.D.
Senior Scientific Liaison 1-301-998-6828 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2597