Powdered Echinacea angustifolia Extract
DEFINITION
Powdered Echinacea angustifolia Extract is prepared from Echinacea angustifolia roots by extraction with hydroalcoholic mixtures or other suitable solvents. The ratio of the starting crude plant material to Powdered Extract is 2:1–8:1. It contains NLT 4.0% and NMT 5.0% of total phenols, calculated on the dried basis as the sum of caftaric acid (C13H12O9), chicoric acid (C22H18O12), chlorogenic acid (C16H18O9), dicaffeoylquinic acids (C25H24O12), and echinacoside (C35H46O20). It contains NLT 0.1% of dodecatetraenoic acid isobutylamides (C16H25NO) on the dried basis.
IDENTIFICATION
• A. Presence of Isobutylalkenylamides
Standard solution A:
Transfer a quantity of USP Powdered Echinacea angustifolia Extract RS to a centrifuge tube, and add chloroform to obtain a solution having a known concentration of about 100 mg/mL. Shake by hand to disperse, sonicate for 5 min, and centrifuge. Use the supernatant.
Standard solution B:
1 mg/mL of 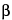 -sitosterol in methanol
-sitosterol in methanol
Sample solution:
100 mg/mL of Powdered Extract in methanol. Allow to stand for 15 min before use.
Adsorbent:
0.25-mm layer of chromatographic silica gel mixture, typically 20 cm long µm (TLC plates)
Application volume:
10 µL
Developing solvent system:
Hexane and ethyl acetate (2:1)
Spray reagent:
Prepare a mixture of glacial acetic acid, sulfuric acid, and p-anisaldehyde (10:5:0.5) in an ice bath
Analysis
Samples:
Standard solution A, Standard solution B, and Sample solution
Develop the chromatograms until the solvent front has moved NLT 12 cm, and dry the plate in a current of air. Examine the plate under UV light at 254 nm, and then spray the plate with Spray reagent, and heat the plate at 100 for 5 min.
for 5 min.
Acceptance criteria
Under UV 254 nm: The chromatogram obtained from the Sample solution shows one main zone at an RF value of about 0.25 due to 2E,4E,8Z,10E-dodecatetraenoic acid isobutylamide and dodeca-2E,4E,8Z,10Z-tetraenoic acid isobutylamide (absent in E. pallida) that corresponds in RF value to that in the chromatogram of Standard solution A.
After treatment with Spray reagent: The chromatogram obtained from the Sample solution shows a zone due to 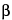 -sitosterol that corresponds in RF value to the principal spot in the chromatogram of Standard solution B. Below this spot, there is a zone due to dodeca-2E,4E,8Z,10E-tetraenoic acid isobutylamide and to dodeca-2E,4E,8Z,10Z-tetraenoic acid isobutylamide that corresponds in RF value to that in the chromatogram of Standard solution A; and below this spot, there are several yellowish zones due to
-sitosterol that corresponds in RF value to the principal spot in the chromatogram of Standard solution B. Below this spot, there is a zone due to dodeca-2E,4E,8Z,10E-tetraenoic acid isobutylamide and to dodeca-2E,4E,8Z,10Z-tetraenoic acid isobutylamide that corresponds in RF value to that in the chromatogram of Standard solution A; and below this spot, there are several yellowish zones due to  ,
,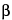 -unsaturated isobutylamides (absent in Echinacea pallida and mostly violet in Echinacea purpurea due to the presence of
-unsaturated isobutylamides (absent in Echinacea pallida and mostly violet in Echinacea purpurea due to the presence of  ,
,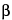 ,
,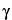 ,
,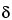 -unsaturated isobutylamides) that are not visible or are very weak when viewed under UV light at 254 nm.
-unsaturated isobutylamides) that are not visible or are very weak when viewed under UV light at 254 nm.
• B.
The retention time of the major peak of the Sample solution corresponds to that of the echinacoside peak of Standard solution A, and the retention time of the peak for 1,3-dicaffeoylquinic acid from the Sample solution corresponds to that of Standard solution A, all peaks as obtained in the test for Content of total phenols.
COMPOSITION
• Content of Total Phenols
Solution A:
Phosphoric acid (0.1 in 100) in water
Solution B:
Acetonitrile
Mobile phase:
See Table 1.
Table 1
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 90 | 10 |
| 13 | 78 | 22 |
| 14 | 60 | 40 |
| 17 | 60 | 40 |
| 17.5 | 90 | 10 |
| 22 | 90 | 10 |
Solvent:
Alcohol and water (7:3)
Standard solution A:
Dissolve USP Powdered Echinacea angustifolia Extract RS in Solvent, shaking and heating in a water bath. Diilute with Solvent to obtain a solution having a known concentration of 1 mg/mL. Pass through a membrane filter having a 0.45-µm or finer pore size.
Standard solution B:
40 µg/mL of USP Chlorogenic Acid RS in Solvent
Sample solution:
Transfer about 60 mg of Powdered Extract, accurately weighed, to an appropriate round-bottom flask equipped with a condenser. Add 25.0 mL of Solvent, and heat under reflux while shaking by mechanical means for 15 min. Centrifuge, or pass through a membrane filter having a 0.45-µm or finer pore size.
Chromatographic system
Mode:
LC
Detector:
UV 330 nm
Column:
4.6-mm × 25-cm; 5-µm packing L1
Column temperature:
35
Flow rate:
1.5 mL/min
Injection size:
5 µL
System suitability
Samples:
Standard solution A and Standard solution B
Suitability requirements
Chromatogram similarity:
The chromatogram obtained from Standard solution A is similar to the Reference Chromatogram for total phenols provided with the USP Powdered Echinacea angustifolia Extract RS.
Resolution:
NLT 1.0 between the 1,3-dicaffeoylquinic acid isomer and echinacoside peaks, Standard solution A
Capacity factor (k¢):
NLT 3.0, Standard solution B
Tailing factor:
NMT 2.0 for the chlorogenic acid peak, Standard solution B
Relative standard deviation:
NMT 2.5% for the chlorogenic acid peak in repeated injections, Standard solution B
Analysis
Samples:
Standard solution A, Standard solution B, and Sample solution
Identify the relevant analytes in the chromatogram obtained from the Sample solution by comparison with the chromatogram obtained from Standard solution A. Measure the areas for the relevant peaks.
Separately calculate the percentage of caftaric acid (C13H12O9), chicoric acid (C22H18O12), chlorogenic acid (C16H18O9), dicaffeoylquinic acids (C25H24O12), and echinacoside (C35H46O20) in the portion of Powdered Extract taken:
Result = (rU/rS) × (CS/CU) × F × 100
| rU | = | = peak response for the relevant analyte from the Sample solution |
| rS | = | = peak response for chlorogenic acid from Standard solution B |
| CS | = | = concentration of USP Chlorogenic Acid RS in Standard solution B (mg/mL) |
| CU | = | = concentration of Powdered Echinacea angustifolia Extract in the Sample solution (mg/mL) |
| F | = | = response factor: chicoric acid, 0.695; dicaffeoylquinic acids, 0.729; caftaric acid, 0.881; chlorogenic acid, 1.000; and echinacoside, 2.220 |
Calculate the percentage of total phenols in the portion of Powdered Extract taken by adding the individual percentages.
Acceptance criteria:
NLT 4.0% and NMT 5.0% of total phenols on the dried basis
• Content of Dodecatetraenoic Acid Isobutylamides
Standard solution A:
Dissolve USP Powdered Echinacea angustifolia Extract RS in methanol, shaking for 1 min, and dilute with methanol to volume to obtain a solution having a known concentration of 1 mg/mL. Pass through a membrane filter having a 0.45-µm or finer pore size.
Standard solution B:
10 µg/mL of USP 2E,4E-Hexadienoic Acid Isobutylamide RS in methanol
Sample solution:
Transfer about 500 mg of Powdered Extract, accuratley weighed, to a 100-mL volumetric flask. Add 80 mL of methanol, and sonicate for 30 min. Dilute with methanol to volume, and pass through a membrane filter having a 0.45-µm or finer pore size.
Mobile phase:
Acetonitrile and water (55:45)
Chromatographic system
Mode:
LC
Detector:
UV 254 nm
Column:
4.6-mm × 25-cm; 5-µm packing L1
Column temperature:
30
Flow rate:
1.5 mL/min
Injection size:
25 µL
System suitability
Samples:
Standard solution A and Standard solution B
Suitability requirements
Chromatogram similarity:
The chromatogram from Standard solution A is similar to the Reference Chromatogram for alkamides provided with USP Powdered Echinacea angustifolia Extract RS.
Resolution:
NLT 1.0 between dodecatetraenoic acid isobutylamide peaks, Standard solution A
Tailing factor:
NMT 2.0 for the 2E,4E-hexadienoic acid isobutylamide peak, Standard solution B
Relative standard deviation:
NMT 2.5% for the 2E,4E-hexadienoic acid isobutylamide peak in repeated injections, Standard solution B
Analysis
Samples:
Standard solution A, Standard solution B, and Sample solution
Identify the peaks due to 2E,4E,8Z,10E-dodecatetraenoic acid isobutylamide and 2E,4E,8Z,10Z-dodecatetraenoic acid isobutylamide in the chromatogram from the Sample solution by comparison with the chromatogram obtained from Standard solution A. Measure the areas for the relevant peaks.
Calculate the percentage of dodecatetraenoic acid isobutylamides in the portion of Powdered Extract taken:
Result = (rU/rS) × (CS/CU) × F × 100
| rU | = | = sum of the peak responses of the relevant analytes from the Sample solution |
| rS | = | = peak response for 2E,4E-hexadienoic acid isobutylamide from Standard solution B |
| CS | = | = concentration of USP 2E,4E-Hexadienoic Acid Isobutylamide RS in Standard solution B (mg/mL) |
| CU | = | = concentration of Powdered Echinacea angustifolia Extract in the Sample solution (mg/mL) |
| F | = | = response factor for 2E,4E-hexadienoic acid isobutylamide, 1.353 |
Acceptance criteria:
NLT 0.1% of dodecatetraenoic acid isobutylamides (C16H25NO) on the dried basis
CONTAMINANTS
• Heavy Metals, Method II  231
231 :
20 ppm
:
20 ppm
• Microbial Enumeration Tests  2021
2021 :
The total bacterial count does not exceed 104 cfu/g and the total combined molds and yeasts count does not exceed 103 cfu/g.
:
The total bacterial count does not exceed 104 cfu/g and the total combined molds and yeasts count does not exceed 103 cfu/g.
• Microbiological Procedures for Absence of Specified Microorganisms  2022
2022 :
Meets the requirements of the tests for absence of Salmonella species, and Escherichia coli.
:
Meets the requirements of the tests for absence of Salmonella species, and Escherichia coli.
• Other Requirements:
It meets the requirements for Botanical Extracts  565
565 , Residual Solvents and Pesticide Residues.
, Residual Solvents and Pesticide Residues.
SPECIFIC TESTS
• Loss on Drying  731
731 :
Dry 1 g at 105
:
Dry 1 g at 105 for 2 h: it loses NMT 5.0%.
for 2 h: it loses NMT 5.0%.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers, in a cool place.
• Labeling:
The label states the Latin binomial and, following the official name, the part of the plant from which the article was prepared. If standardized by the content of alkamides, label it to indicate the targeted content of dodecatraenoic acid isobutylamides. The label bears a statement indicating that Echinacea angustifolia may cause rare allergic reactions, rashes, or aggravate asthma. It meets the requirements for Botanical Extracts  565
565 , Labeling.
, Labeling.
• USP Reference Standards  11
11
USP Powdered Echinacea angustifolia Extract RS
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Maged H. Sharaf, Ph.D.
Principal Scientific Liaison 1-301-816-8318 |
(DS2010) Monographs - Dietary Supplements |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1269
Pharmacopeial Forum: Volume No. 30(2) Page 554
