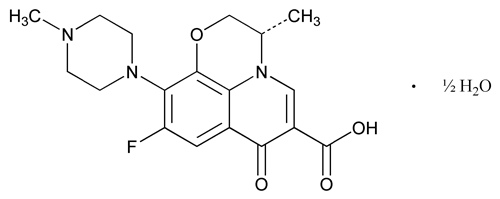
Levofloxacin
C18H20FN3O4·½H2O 370.38
7H-Pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid, 9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-hydrate (2:1), (S)-;
( )-(S)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid, hemihydrate
)-(S)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid, hemihydrate


 [138199-71-0].
[138199-71-0].
Anhydrous

 [100986-85-41].
[100986-85-41].
(lee'' voe flox' a sin).
C18H20FN3O4·½H2O 370.38
7H-Pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid, 9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-hydrate (2:1), (S)-;
(
Anhydrous
DEFINITION
Levofloxacin contains NLT 98.5% and NMT 102.0% of C18H20FN3O4, calculated on the anhydrous basis.
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
Solution A:
8.5 g/L of ammonium acetate, 1.25 g/L of cupric sulfate, pentahydrate, and 1.3 g/L of l-isoleucine in water
Mobile phase:
Methanol and Solution A (3:7)
Standard solution:
1 mg/mL of USP Levofloxacin RS in Mobile phase
Sample solution:
1 mg/mL of Levofloxacin in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 360 nm
Column:
4.6-mm × 25-cm; 5-µm packing L1
Column temperature:
45
Flow rate:
0.8 mL/min
Injection size:
25 µL
System suitability
Sample:
Standard solution
Suitability requirements
Tailing factor:
0.5–1.5
Relative standard deviation:
NMT 1.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C18H20FN3O4 in the portion of Levofloxacin taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of Levofloxacin from the Sample solution |
| rS | = | = peak response of levofloxacin from the Standard solution |
| CS | = | = concentration of USP Levofloxacin RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Levofloxacin in the Sample solution (mg/mL) |
Acceptance criteria:
98.5%–102.0% on the anhydrous basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.2%. Use a platinum crucible.
:
NMT 0.2%. Use a platinum crucible.
• Heavy Metals, Method II  231
231 :
NMT 10 ppm
:
NMT 10 ppm
Organic Impurities
• Procedure
Solution A, Mobile phase, Sample solution, and Chromatographic system:
Proceed as directed in the Assay.
System suitability solution:
1 mg/mL of USP Levofloxacin RS in Mobile phase
Sensitivity solution:
0.3 µg/mL of USP Levofloxacin RS in Mobile phase
System suitability
Samples:
System suitability solution and Sensitivity solution
Suitability requirements
Relative standard deviation:
NMT 1.0%, System suitability solution
Signal-to-noise ratio:
NLT 10, Sensitivity solution
Analysis
Sample:
Sample solution
Calculate the percentage of each individual impurity in the portion of Levofloxacin taken:
Result = (rU/rS) × (1/F) × 100
| rU | = | = peak area response of each impurity |
| rS | = | = peak area response of levofloxacin |
| F | = | = relative response factor (see Impurity Table 1) |
Acceptance criteria
Individual impurities:
See Impurity Table 1.
Total impurities:
NMT 0.5%. [Note—Do not include the d-isomer in the calculation for Total impurities. ]
Impurity Table 1
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| N-Desmethyl levofloxacina | 0.47 | 1.0 | 0.3 |
| Diamine derivativeb | 0.52 | 0.9 | 0.3 |
| Levofloxacin N-oxidec | 0.63 | 1.1 | 0.3 |
| 9-Desfluoro levofloxacind | 0.73 | 1.0 | 0.3 |
| Levofloxacin | 1.0 | — | — |
| d-Isomere | 1.23 | 1.0 | 0.8 |
| Any unknown impurity |
— | 1.0 | 0.1 |
|
a
(S)-9-Fluoro-2,3-dihydro-3-methyl-10-(piperazin-1-yl)-7-oxo-7H-pyrido[1,2,3-de][1,4]benzoxazine-6-carboxylic acid.
b
(S)-9-Fluoro-2,3-dihydro-3-methyl-10-[2-(methylamino)ethylamino]-7-oxo-7H-pyrido[1,2,3-de][1,4]benzoxazine-6-carboxylic acid.
c
(S)-4-(6-Carboxy-9-fluoro-2,3-dihydro-3-methyl-7-oxo-7H-pyrido-[1,2,3-de][1,4]benzoxazine-10-yl)-1-methyl-piperazine-1-oxide.
d
(S)-2,3-Dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de][1,4]benzoxazine-6-carboxylic acid.
e
(R)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de][1,4]benzoxazine-6-carboxylic acid.
|
|||
SPECIFIC TESTS
• Optical Rotation, Specific Rotation  781S
781S
Solvent:
Methanol
Sample solution:
5 mg/mL in Solvent
Acceptance criteria:
 92
92 to
to  106
106 , at 20
, at 20
• Water Determination, Method Ia  921
921 :
2.1%–2.7%
:
2.1%–2.7%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight and light-resistant containers. Store at room temperature.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Behnam Davani, Ph.D., M.B.A.
Senior Scientific Liaison 1-301-816-8394 |
(SM12010) Monographs - Small Molecules 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3671
Pharmacopeial Forum: Volume No. 35(6) Page 1459