Minocycline Periodontal System
DEFINITION
Minocycline Periodontal System is an extended-release formulation of Minocycline Hydrochloride containing the equivalent of NLT 90.0% and NMT 120.0% of the labeled amount of minocycline (C23H27N3O7).
IDENTIFICATION
• A. Ultraviolet Absorption  197U
197U
Wavelength range:
250–450 nm
Standard stock solution:
Transfer USP Minocycline Hydrochloride RS to a suitable volumetric flask. Dissolve first in dimethylformamide, using about 20% of the final volume, then dilute with water to volume, and mix to obtain a solution having a known concentration of about 0.48 mg/mL of minocycline.
Standard solution:
0.024 mg/mL minocycline hydrochloride from Standard stock solution in water
Sample stock solution:
Transfer Minocycline Periodontal System equivalent to 12 mg of minocycline hydrochloride to a 25-mL volumetric flask. Add 5.0 mL of dimethylformamide, and mix to dissolve. Dilute with water to volume and filter.
Sample solution:
0.024 mg/mL minocycline hydrochloride from Sample stock solution in water
Acceptance criteria:
The Sample solution exhibits maxima at the same wavelengths as the Standard solution, concomitantly measured.
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
Mobile phase:
Dimethylformamide, 0.2 M ammonium oxalate, and 0.1 M edetate disodium (25:55:20), adjusted with 0.4 M aqueous tetrabutylammonium hydroxide solution to a pH of 6.3 ± 0.2
Diluent:
Dimethylformamide and methanol (1:1)
System suitability solution:
Prepare a solution in water containing 2 mg/mL USP Minocycline Hydrochloride RS. Heat over a steam bath for 60 min. To one part of this solution add four parts of Mobile phase and mix. Refrigerate the solution immediately after preparation and during analysis, using a refrigerated autosampler.
Standard solution:
0.4 mg/mL of minocycline from USP Minocycline Hydrochloride RS in Diluent. Refrigerate the solution immediately after preparation and during analysis, using a refrigerated autosampler. [Note—Use low-actinic glassware. ]
Sample solution:
Mix the contents of NLT 10 dispensing units of Minocycline Periodontal System. Transfer a portion of the mixture, equivalent to 10 mg of minocycline, into a 25-mL volumetric flask. Add Diluent and sonicate for 2–5 min, or until the sample is dissolved. Dilute with Diluent to volume, and mix to obtain a solution having a nominal concentration of 0.4 mg/mL of minocycline, based on the label claim. Refrigerate the solution immediately after preparation and during analysis, using a refrigerated autosampler. [Note—Use low-actinic glassware. ]
Chromatographic system
Mode:
LC
Detector:
UV 280 nm
Guard column:
4.6-mm × 3-cm; 10-µm packing L7
Column:
4.6-mm × 15-cm; 5-µm packing L7
Flow rate:
2 mL/min
Autosampler temperature:
5
Injection size:
10 µL
System suitability
Samples:
System suitability solution and Standard solution
[Note—The relative retention times of epiminocycline and minocycline are 0.81 and 1, respectively. ]
Suitability requirements
Resolution:
NLT 2.0 between epiminocycline and minocycline, System suitability solution
Tailing factor:
NMT 2.0 for the minocycline peak, System suitability solution
Relative standard deviation:
NMT 2.0% for the minocycline peak, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of minocycline (C23H27N3O7) in the portion of the Minocycline Periodontal System taken:
Result = (rU/rS) × (CS/CU) × P × F × 100
| rU | = | = peak response of minocycline from the Sample solution |
| rS | = | = peak response of minocycline from the Standard solution |
| CS | = | = concentration of USP Minocycline Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of minocycline in the Sample solution (mg/mL) |
| P | = | = potency of minocycline in USP Minocycline Hydrochloride RS (µg/mg) |
| F | = | = conversion factor, 0.001 mg/µg |
Acceptance criteria:
90.0%–120.0%
PERFORMANCE TESTS
• Dissolution
Medium:
6.9 g/L of monobasic sodium phosphate monohydrate in water, adjusted with phosphoric acid to a pH of 4.2. This solution is stable for 10 days.
Apparatus:
Tube rotator. [Note—Suitable equipment is available as Labquake® tube rotator, catalog number 400110. ]
0.1 M Edetate disodium:
37.2 g/L of edetate disodium in water
0.2 M Ammonium oxalate:
28.4 g/L of ammonium oxalate in water
Mobile phase:
Mix 310 mL of 0.1 M Edetate disodium and 500 mL of 0.2 M Ammonium oxalate, adjust with 0.4 M aqueous tetrabutylammonium hydroxide to a pH of 6.2, and add 175 mL of dimethylformamide. The injector wash solution is a mixture of dimethylformamide and water (25:75).
Standard stock solution:
0.11 mg/mL of USP Minocycline Hydrochloride RS in Medium
Standard solutions:
Dilute the Standard stock solution with Medium to obtain solutions with final concentrations of 0.088 mg/mL, 0.0528 mg/mL, 0.0352 mg/mL, 0.022 mg/mL, and 0.0176 mg/mL.
System suitability solution:
Transfer 10 mg of USP Minocycline Hydrochloride RS to a 50-mL beaker. Add 5 mL of water and heat on a steam bath for 60 min. Add 20 mL of Medium or Mobile phase, and mix well. Store at 5 .
.
Sample solution:
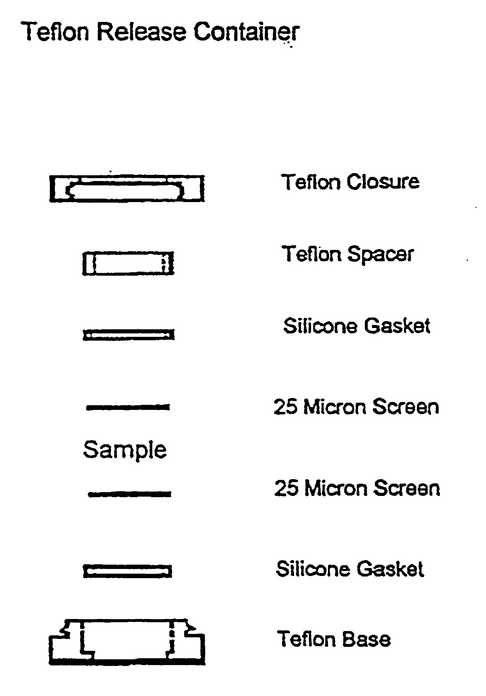
Use borosilicate glass tubes, 25 mm outside diameter and 15 cm long. Close the tubes with a snap type cell with a Teflon prong consisting of a Teflon closure and holder that snap together, two 25-µm stainless steel screens, two silicone gaskets, and a Teflon spacer (see Figure 1). Prepare six tubes as follows: partially assemble a release tube and tare its weight; dispense one dose of Minocycline Periodontal System into a partially assembled release cell (see Figure 1); record the sample weight in mg; assemble the cell so that the sample is enclosed between the two 25-µm screens; close the cells and place each one of them into separate glass tubes containing 10 mL of Medium previously equilibrated at 37 ; add the Teflon prong, and cap the tube with Teflon faced rubber-lined caps; seal with Teflon tape. Place the tubes in the tube rotator. Place the tube rotator in a convection incubator that is maintained at 37
; add the Teflon prong, and cap the tube with Teflon faced rubber-lined caps; seal with Teflon tape. Place the tubes in the tube rotator. Place the tube rotator in a convection incubator that is maintained at 37 . Allow the tubes to rotate for 4 h. Remove the solution under test, and add 10 mL of Medium previously equilibrated at 37
. Allow the tubes to rotate for 4 h. Remove the solution under test, and add 10 mL of Medium previously equilibrated at 37 . Replace the tubes in the apparatus and rotate for 20 h (24 h total). Repeat the sampling procedure after 24 h (48 h total), and after another 24 h (72 h total).
. Replace the tubes in the apparatus and rotate for 20 h (24 h total). Repeat the sampling procedure after 24 h (48 h total), and after another 24 h (72 h total).
Chromatographic system
Mode:
LC
Detector:
UV 280 nm
Guard column:
4.6-mm × 3-cm; 10-µm packing L7
Column:
4.6-mm × 3.3-cm; 5-µm packing L1
Flow rate:
1.5 mL/min
Autosampler temperature:
5
Injection size:
20 µL for the 4 and 24 h time points; 50 µL for the 48 and 72 h time points
Suitability requirements
Samples:
System suitability solution and Standard solutions
Resolution:
NLT 2.0 between epiminocycline and minocycline. Inject 20 µL of the System suitability solution.
Tailing factor:
NMT 2.0. Inject 20 µL of the System suitability solution.
Relative standard deviation:
NMT 2.0% for the minocycline peak, any of the Standard solutions
Analysis:
Construct a calibration curve for each sampling interval by plotting the concentration of the Standard solutions versus peak area. Calculate the slopes and y-intercepts using linear regression analysis.
Calculate the release rate of minocycline:
Resulti = [(rUi  yi)/Si] × 10/( i × W × A)
yi)/Si] × 10/( i × W × A)
| i | = | = sampling time, 4, 24, 48, 72 h |
| rUi | = | = peak response from each of the Standard solutions at time i |
| yi | = | = y-intercept of the calibration curve at sampling time i |
| Si | = | = slope of the calibration curve at sampling time i |
| W | = | = weight of the sample (mg) |
| A | = | = amount of minocycline in the sample (mg/mg of sample) as determined in the Assay |
Tolerances
| Time (h) |
Release Rate (µg/h) Average of 6 Measurements |
|---|---|
| 0–4 | NLT 25 |
| 4–24 | NLT 1.0 |
| 24–48 | NLT 0.2 |
| 48–72 | NLT 0.05 |
• Uniformity of Dosage Units  905
905 :
Meet the requirements
:
Meet the requirements
IMPURITIES
Organic Impurities
• Procedure
Mobile phase, Diluent, System suitability solution, Standard solution, Sample solution, Chromatographic system, and System suitability:
Proceed as directed in the Assay.
Analysis
Sample:
Sample solution
Calculate the percentage of each related compound in the portion of Minocycline Periodontal System taken:
Result = (rU/rT) × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rT | = | = sum of the peak responses from the Sample solution. [Note—Exclude peaks eluting in the solvent front. ] |
Acceptance criteria
Individual impurities:
NMT 6.0% of epiminocycline
Total impurities (excluding epiminocycline):
NMT 3.5%
SPECIFIC TESTS
• Water Determination, Method I  921
921 :
NMT 5.0%
:
NMT 5.0%
• Microbial Enumeration Tests  61
61 and Tests for Specified Organisms
and Tests for Specified Organisms  62
62 :
The total aerobic microbial count does not exceed 1000 cfu/g; the total combined molds and yeasts count does not exceed 100 cfu/g; and the product meets the requirements of the test for the absence of Escherichia coli.
:
The total aerobic microbial count does not exceed 1000 cfu/g; the total combined molds and yeasts count does not exceed 100 cfu/g; and the product meets the requirements of the test for the absence of Escherichia coli.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in a tight, light-resistant container. Store at controlled room temperature.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ahalya Wise, M.S.
Senior Scientific Liaison 1-301-816-8161 |
(SM12010) Monographs - Small Molecules 1 |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3929
Pharmacopeial Forum: Volume No. 36(2) Page 420