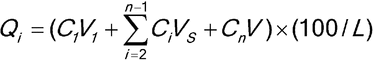Tamsulosin Hydrochloride Capsules
DEFINITION
Tamsulosin Hydrochloride Capsules contain NLT 90.0% and NMT 110.0% of the labeled amount of tamsulosin hydrochloride (C20H28N2O5S·HCl).
IDENTIFICATION
• The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure 1
Solution A:
Dilute 20 mL of hydrochloric acid with water to 1000 mL.
Solution B:
Dissolve 8.7 mL of perchloric acid and 3.0 g of sodium hydroxide in 1900 mL of water. Adjust with 1 N sodium hydroxide to a pH of 2.0, and add sufficient water to make 2000 mL.
Mobile phase:
Acetonitrile and Solution B (3:7)
Internal standard solution:
0.4 mg/mL of propylparaben in a mixture of acetonitrile and water (3:7)
Standard stock solution:
0.5 mg/mL of USP Tamsulosin Hydrochloride RS in a mixture of acetonitrile and water (3:7)
Standard solution:
Transfer 2.0 mL of the Standard stock solution to a suitable container, add 5.0 mL of Internal standard solution, and add Mobile phase to make 40 mL.
Sample solution:
Weigh the contents of NLT 20 Capsules. Mix the contents, and transfer a weighed portion of the powder, equivalent to about 1 mg of tamsulosin hydrochloride based on the label claim, into a Teflon-lined, screw-capped centrifuge tube. Place approximately 100 glass balls with a diameter of about 5 mm into the tube, add 20 mL of 0.05 N sodium hydroxide, heat at 50 for 10 min, and shake well for 30 min. Add 15 mL of a mixture of acetonitrile and Solution A (2:1) to the solution, and shake well. Add 5.0 mL of the Internal standard solution, and shake well. Centrifuge at 1500 rpm for 10 min, and use the supernatant, passing it if necessary through a membrane filter of pore size 0.5 µm or smaller.
for 10 min, and shake well for 30 min. Add 15 mL of a mixture of acetonitrile and Solution A (2:1) to the solution, and shake well. Add 5.0 mL of the Internal standard solution, and shake well. Centrifuge at 1500 rpm for 10 min, and use the supernatant, passing it if necessary through a membrane filter of pore size 0.5 µm or smaller.
Chromatographic system
Mode:
LC
Detector:
UV 225 nm
Column:
4.0-mm × 15-cm or 4.6-mm × 15-cm; 5-µm packing L1
Column temperature:
40
Flow rate:
1.0 mL/min for the 4.0-mm column and 1.3 mL/min for the 4.6-mm column. [Note—The flow rate can be adjusted as needed to achieve a recommended retention time of approximately 6 min for tamsulosin. ]
Injection size:
10 µL
System suitability
Sample:
Standard solution
Suitability requirements
Resolution:
NLT 12 between tamsulosin and propylparaben. [Note—The elution order is tamsulosin hydrochloride followed by propylparaben. ]
Relative standard deviation:
NMT 2.0%, for the ratios of the peak areas for tamsulosin and the internal standard
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of the labeled amount of tamsulosin hydrochloride (C20H28N2O5S·HCl) in the portion of Capsules taken:
Result = (RU/RS) × (CS × VS/W) × 100
| RU | = | = ratio of the peak areas for tamsulosin and the internal standard from the Sample solution |
| RS | = | = ratio of the peak areas for tamsulosin and the internal standard from the Standard solution |
| CS | = | = concentration of USP Tamsulosin Hydrochloride RS in the Standard stock solution (mg/mL) |
| VS | = | = volume of the Standard stock solution taken to prepare the Standard solution (mL) |
| W | = | = amount of tamsulosin hydrochloride, based on the label claim, taken to prepare the Sample solution (mg) |
Acceptance criteria:
90.0%–110.0%
• Procedure 2:
Use this Procedure for Capsules labeled to meet the requirements of Dissolution Test 2.
Solution B and Mobile phase:
Proceed as directed for Procedure 1.
Buffer:
Dissolve 3.4 g of monobasic potassium phosphate in 1 L of water. Adjust with 2 N sodium hydroxide to a pH of 5.80 ± 0.05.
Standard solution:
Prepare a solution containing 1.2 mg/mL of USP Tamsulosin Hydrochloride RS in methanol. Dilute with Buffer to obtain a solution containing 3.2 µg/mL.
Sample solution:
Weigh the contents of NLT 20 Capsules. Mix the contents, and transfer a weighed portion of the Capsule contents, equivalent to 1.6 mg of tamsulosin hydrochloride, into a 100-mL volumetric flask. Add 20 mL of methanol, stir for 30 min, sonicate for 30 min, and stir again for 30 min. Add 40 mL of methanol, sonicate for another 30 min, and stir for another 60 min. Dilute with methanol to volume, mix well, and allow the solution to stand for 5 min. Dilute 5 mL of this solution with Buffer to 25 mL, and allow the solution to stand for 5 min. Pass through a PVDF filter of 0.45-µm pore size, discarding the first 5 mL of the filtrate.
Chromatographic system:
Proceed as directed for Procedure 1, except inject 50 µL.
System suitability
Sample:
Standard solution
Suitability requirements
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of the labeled amount of tamsulosin hydrochloride (C20H28N2O5S·HCl) in the portion of Capsules taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of the Standard solution (mg/mL) |
| CU | = | = nominal concentration of the Sample solution (mg/mL) |
Acceptance criteria:
90.0%–110.0%
• Procedure 3:
Use this Procedure for Capsules labeled to meet the requirements of Dissolution Test 3.
Solution B and Mobile phase:
Proceed as directed for Procedure 1.
Buffer:
Dissolve 6.9 g of monobasic sodium phosphate monohydrate in 1 L of water, and adjust with 5 N sodium hydroxide to a pH of 7.2 ± 0.05.
Standard solution:
Prepare a solution containing 0.5 mg/mL of USP Tamsulosin Hydrochloride RS in a mixture of methanol and water (1:1), and dilute a portion of this solution with methanol to obtain a solution containing 0.03 mg/mL.
Sample solution:
Weigh the contents of NLT 20 Capsules. Mix the contents, and transfer a weighed portion of the Capsule contents, equivalent to 0.8 mg of tamsulosin hydrochloride, into a 25-mL volumetric flask. Add 5 mL of Buffer, shake for 15 min, add 10 mL of methanol, shake for 1 h, and dilute with methanol to volume. Pass through a suitable filter of 0.45-µm pore size.
Chromatographic system:
Proceed as directed for Procedure 1, except inject 20 µL.
System suitability and Analysis:
Proceed as directed for Procedure 2.
Acceptance criteria:
90.0%–110.0%
• Procedure 4:
For Capsules labeled to meet the requirements of Dissolution Test 4, proceed as directed for Procedure 1.
• Procedure 5:
Use this Procedure for Capsules labeled to meet the requirements of Dissolution Test 5.
Solution B, Mobile phase, and Chromatographic system:
Proceed as directed for Procedure 1.
Diluent:
Methanol and water (75:25)
Standard solution:
Prepare a solution containing 0.016 mg/mL of USP Tamsulosin Hydrochloride RS in Diluent.
Sample solution:
Transfer the contents of 10 Capsules (equivalent to 4 mg of tamsulosin hydrochloride) into a 250-mL volumetric flask. Add 200 mL of Diluent, stir, and sonicate simultaneously for at least 2 h. Cool, and dilute with Diluent to volume. Pass through a suitable filter.
System suitability and Analysis:
Proceed as directed for Procedure 2.
Acceptance criteria:
90.0%–110.0%
• Procedure 6:
Use this Procedure for Capsules labeled to meet the requirements of Dissolution Test 6.
Solution B and Mobile phase:
Proceed as directed for Procedure 1.
Buffer:
Dissolve 6.8 g of monobasic potassium phosphate and 0.9 g of sodium hydroxide in 1 L of water. Adjust with sodium hydroxide solution to a pH of 6.8 ± 0.05.
Diluent A:
Acetonitrile and Buffer (1:1)
Diluent B:
Acetonitrile and Solution B (1:1)
Standard solution:
Prepare a solution containing 0.4 mg/mL of USP Tamsulosin Hydrochloride RS in Diluent A, using sonication as necessary. Dilute 2 mL of this solution with Diluent B to 100 mL.
Sample solution:
Weigh the contents of NLT 20 Capsules. Mix the contents, and transfer a weighed portion of the Capsule contents, equivalent to 4 mg of tamsulosin hydrochloride, into a 100-mL volumetric flask. Add 60 mL of Diluent A, and sonicate with intermittent shaking to disperse the pellets completely. Cool, and dilute with Diluent A to volume. Centrifuge, transfer 5 mL of supernatant solution to a 25-mL volumetric flask, and dilute with Diluent B to volume. Pass through a nylon membrane filter of 0.45-µm pore size.
Chromatographic system:
Proceed as directed for Procedure 1, except inject 20 µL.
System suitability and Analysis:
Proceed as directed for Procedure 2.
Acceptance criteria:
90.0%–110.0%
• Procedure 7:
Use this Procedure for Capsules labeled to meet the requirements of Dissolution Test 7.
Solution B and Mobile phase:
Proceed as directed for Procedure 1.
Buffer:
Dissolve 76 g of tribasic sodium phosphate in 1 L of water.
Diluent:
0.1 N hydrochloric acid and Buffer (3:1), adjusted with diluted hydrochloric acid or sodium hydroxide solution to a pH of 7.0
Standard solution:
0.0016 mg/mL of USP Tamsulosin Hydrochloride RS in Diluent
Sample solution:
Weigh the contents of NLT 20 Capsules. Mix the contents, and transfer a weighed portion of the Capsule contents, equivalent to 0.4 mg of tamsulosin hydrochloride, into a 250-mL volumetric flask, and dilute with Diluent to volume. Stir for 24 h by mechanical means at 40 protected from light. Pass through a suitable filter of 0.45-µm pore size.
protected from light. Pass through a suitable filter of 0.45-µm pore size.
Chromatographic system:
Proceed as directed for Procedure 1, except inject 50 µL.
System suitability and Analysis:
Proceed as directed for Procedure 2.
Acceptance criteria:
90.0%–110.0%
• Procedure 8:
Use this Procedure for Capsules labeled to meet the requirements of Dissolution Test 8.
Solution B, Mobile phase, and Chromatographic system:
Proceed as directed for Procedure 1.
Standard solution:
Prepare a solution containing 1.0 mg/mL of USP Tamsulosin Hydrochloride RS in methanol. Dilute 5 mL of this solution with Mobile phase to 20 mL.
Sample solution:
Weigh the contents of NLT 20 Capsules. Mix the contents, and transfer a weighed portion of the Capsule contents, equivalent to 2.5 mg of tamsulosin hydrochloride, into a 100-mL volumetric flask. Add 25 mL of 0.1 N sodium hydroxide, and sonicate for 30 min. Add 30 mL of Mobile phase, and shake by mechanical means for 30 min. Centrifuge, and pass through a PVDF membrane filter of 0.45-µm pore size, discarding the first few mL of the filtrate.
System suitability and Analysis:
Proceed as directed for Procedure 2.
Acceptance criteria:
90.0%–110.0%
• Procedure 9:
For Capsules labeled to meet the requirements of Dissolution Test 9, proceed as directed for Procedure 1.
PERFORMANCE TESTS
• Dissolution  711
711
Test 1
Acid stage medium:
Dissolve 2.0 g of sodium chloride in 5.7 mL of hydrochloric acid, and add water to make up to 1000 mL. To 500 mL of this fluid add 1 mL of polysorbate 80 aqueous solution (3 g in 200 mL of water) just before the test; 500 mL.
Buffer stage medium:
Phosphate buffer, pH 7.2 (dissolve 6.8 g of monobasic potassium phosphate in 250 mL of water, add 90 mL of 0.2 N sodium hydroxide and 500 mL of water, adjust with 0.2 N sodium hydroxide or 0.2 N hydrochloric acid to a pH of 7.2 ± 0.05, and dilute with water to volume); 500 mL
Apparatus 2:
100 rpm, with sinker (see Figure 2a in Dissolution  711
711 )
)
Times:
2, 3, and 8 h
Analysis:
Perform the test using Acid stage medium. At 2 h after the start of the test, withdraw 10.0 mL of the solution under test (T1). Drain the Acid stage medium immediately by suction through a tube capped with a 60-mesh stainless wire screen. Rinse the drain tube while adding the Buffer stage medium previously warmed, and continue the test. At 3 h after the start of the test (1 h after replacement of the Medium), withdraw 10.0 mL of the solution under test (T2), replace the same volume with warmed Buffer stage medium, and continue the test. At 8 h after the start of the test (6 h after the replacement of the Medium), withdraw 10.0 mL of the solution under test (T3).
Internal standard solution:
0.008 mg/mL of propylparaben in acetonitrile and water (3:7)
Standard solution:
Prepare a solution containing 0.5 mg/mL of USP Tamsulosin Hydrochloride RS in acetonitrile and water (3:7). Transfer 4.0 mL of this solution to a 100-mL volumetric flask, and dilute with Acid stage medium to volume. Transfer 4.0 mL of this dilution to another 100-mL volumetric flask, and dilute with Acid stage medium to volume. This solution has a known concentration (CS) of about 0.8 µg/mL of tamsulosin hydrochloride. Transfer 10.0 mL of this last dilution to a test tube, and add 2.0 mL of the Internal standard solution.
Sample solutions:
Add 2.0 mL of the Internal standard solution to T1, mix well, and pass through a suitable filter of 0.5-µm pore size, discarding the first 5 mL. Add 1.0 mL of 0.5 N hydrochloric acid and 2.0 mL of Internal standard solution to T2, mix well, and pass through a suitable filter of 0.5-µm pore size, discarding the first 5 mL. Add 1.0 mL of 0.5 N hydrochloric acid and 2.0 mL of Internal standard solution to T3. Mix well, and pass through a suitable filter of 0.5-µm pore size, discarding the first 5 mL.
Chromatographic system:
Proceed as directed in the Assay, Procedure 1, but using the Standard solution described for Dissolution, and inject 250 µL instead of 10 µL.
Calculate the ratios (RT1, RT2, RT3, and RS) of the peak area of tamsulosin to that of the internal standard for all Sample solutions and the Standard solution. Calculate the percentage of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at each of the following time points.
At 2 h:
D1 = [(CS ×V)/L] × (RT1/RS) × 100
At 3 h:
D2 = [(CS × V)/L] × [(RT2/RS) + RT1/RS)] × 100
At 8 h:
D3 = [(CS × V)/L] × [(RT3/RS) + (RT2/RS × VT2/V) + (RT1/RS)] × 100
| CS | = | = concentration of the Standard solution |
| V | = | = volume of Medium, 500 mL |
| L | = | = label claim (mg/Capsule) |
| VT2 | = | = volume of the withdrawn aliquot of T2, 10 mL |
Tolerances:
See Table 1.
Table 1
| Time (h) |
Amount Dissolved |
|---|---|
| 2 | 13%–34% |
| 3 | 47%–68% |
| 8 | NLT 80% |
The percentages of the labeled amount of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at the times specified conform to Acceptance Table 2 in Dissolution  711
711 .
.
Test 2:
If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 2.
Acid stage medium:
0.003% polysorbate 80, pH 1.2 (dilute 8.5 mL of hydrochloric acid with water to 900 mL, add 0.03 mL of polysorbate 80, adjust with 0.2 N sodium hydroxide or 0.2 N hydrochloric acid to a pH of 1.2 ± 0.05, and dilute with water to 1000 mL); 500 mL
Buffer stage medium:
Phosphate buffer, pH 7.2 (proceed as directed for Test 1); 500 mL
Apparatus 2:
100 rpm, with sinkers1
Times:
2 h for the Acid stage medium and 6 h for the Buffer stage medium; 8 h total test time
Analysis:
Perform the test using Acid stage medium. At 2 h after the start of the test, withdraw a sample of the solution under test. Carefully discard the Acid stage medium and replace it with the Buffer stage medium previously warmed, and continue the test. At 6 h after the replacement of the Medium, withdraw a sample of the solution under test.
Standard stock solution:
Transfer about 25.0 mg of USP Tamsulosin Hydrochloride RS to a 50-mL volumetric flask. Add 25 mL of methanol. Dilute with Buffer stage medium to volume. Sonicate until dissolved.
Standard solution:
Dilute the Standard stock solution with Buffer stage medium to obtain a final concentration of 0.8 µg/mL.
Sample solution:
Pass a portion of the solution under test through a suitable filter.
Buffer solution:
3.4 g/L of monobasic potassium phosphate in water. Adjust with 2 N sodium hydroxide to a pH of 5.80 ± 0.05.
Mobile phase:
Acetonitrile and Buffer solution (1:3)
Chromatographic system
Mode:
LC
Detector:
UV 225 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Flow rate:
1.0 mL/min
Injection size:
50 µL
System suitability
Sample:
Standard solution
Suitability requirements
Column efficiency:
NLT 2000
Tailing factor:
NMT 2.0
Relative standard deviation:
NMT 2.0%
Calculate the concentration of tamsulosin hydrochloride (C20H28N2O5S·HCl) in the Medium at each time point (Ci).
At 2 h:
C1 = (rU/rS) × CS
At 6 h:
C2 = (rU/rS) × CS
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of the Standard solution (mg/mL) |
Calculate the percentage of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at each time point, (Qi).
At 2 h:
Q1 = (C1 × V) × 100/L
At 6 h:
Q2 = (C1 + C2) × (V/L) × 100
| V | = | = volume of Medium (mL) |
| L | = | = label claim (mg/Capsule) |
Tolerances:
NMT 25% of the labeled amount of tamsulosin hydrochloride (C20H28N2O5S·HCl) is dissolved in 2 h. NLT 85% of the labeled amount of tamsulosin hydrochloride (C20H28N2O5S·HCl) is dissolved in 8 h.
Test 3:
If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 3.
Acid stage
Acid stage medium:
0.003% polysorbate 80, pH 1.2 (proceed as directed for Test 2); 500 mL
Apparatus 2:
100 rpm, with sinkers
Time:
2 h
Analysis:
Pass a portion of the solution under test through a suitable filter. Leave the remaining Acid stage medium in the vessel and proceed with the Buffer stage.
Tolerances:
NMT 10% (Q) of the labeled amount of tamsulosin hydrochloride (C20H28N2O5S·HCl) is dissolved.
Buffer stage
Buffer stage concentrate:
0.1 M phosphate buffer, pH 11.6 (138 g of sodium phosphate monohydrate and 320 mL of 5 N sodium hydroxide in 10 L of water. Adjust with 5 N sodium hydroxide to a pH of 11.6 ± 0.05.)
Buffer stage medium:
Add 500 mL of the Buffer stage concentrate to the remaining Acid stage medium in each vessel. The final pH is about 7.2.
Apparatus 2:
100 rpm, with sinkers
Time:
3 and 8 h, including the 2 h in the Acid stage medium
Standard stock solution:
0.5 mg/mL of USP Tamsulosin Hydrochloride RS in water and methanol (1:1)
Standard solution:
Dilute the Standard stock solution with Buffer stage medium to obtain a final concentration of 0.3 µg/mL.
Sample solution:
Pass a portion of the solution under test through a suitable filter.
pH 5.5 buffer solution:
6.8 g/L of monobasic potassium phosphate in water. Adjust with 2 N sodium hydroxide to a pH of 5.5 ± 0.05.
Mobile phase:
Acetonitrile and pH 5.5 buffer solution (2:3)
Chromatographic system
Mode:
LC
Detector:
UV 275 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Column temperature:
30
Flow rate:
1 mL/min
Injection size:
100 µL
System suitability
Sample:
Standard solution
Suitability requirements
Tailing factor:
NMT 2.0
Relative standard deviation:
NMT 2.5%
Calculate the concentration of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at each time point (Ci).
At 3 h:
C1 = (rU/rS) × CS
At 8 h:
C2 = (rU/rS) × CS
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of the Standard solution (mg/mL) |
Calculate the cumulative percentage of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at each time point (Qi):
| V1 | = | = volume of Acid stage medium, 500 mL |
| VS | = | = volume of sample taken (mL) |
| Cn | = | = concentration of tamsulosin hydrochloride at each time point |
| V | = | = volume of Buffer stage medium, 1000 mL |
| L | = | = label claim (mg/Capsule) |
Tolerances:
See Table 2.
Table 2
| Time (h) |
Amount Dissolved |
|---|---|
| 3 | 65%–85% |
| 8 | NLT 80% |
The percentages of the labeled amount of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at the times specified conform to Acceptance Table 2 in Dissolution  711
711 .
.
Test 4:
If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 4.
Acid stage medium:
0.003% polysorbate 80, pH 1.2 (proceed as directed for Test 2); 500 mL
Buffer stage medium:
Phosphate buffer, pH 7.2 (proceed as directed for Test 1); 500 mL
Apparatus 2:
100 rpm, with sinkers
Times:
2 h for the Acid stage medium, and 3 and 8 h for the Buffer stage medium (including the 2 h in the Acid stage medium)
Analysis:
Perform the test using the Acid stage medium. At 2 h after the start of the test, withdraw a sample of the solution under test. Carefully discard the Acid stage medium, replace it with the Buffer stage medium previously warmed, and continue the test. At 1 h and 6 h after the replacement of the Medium, withdraw a sample of the solution under test. Replace the volume of Medium withdrawn with the same volume of Buffer stage medium, previously warmed.
50 mM sodium perchlorate solution:
Dissolve 7.0 g of monohydrate sodium perchlorate in 1 L of water, and add 5 mL of phosphoric acid.
Mobile phase:
50 mM sodium perchlorate solution and acetonitrile (3:2)
Acid stage standard stock solution:
Transfer 20 mg of USP Tamsulosin Hydrochloride RS to a 500-mL volumetric flask, add 25 mL of methanol, and sonicate until dissolved. Dilute with Acid stage medium to volume.
Acid stage standard solution:
Dilute the Acid stage standard stock solution with Acid stage medium to obtain a final concentration of about 0.08 µg/mL.
Buffer stage standard stock solution:
Transfer 20 mg of USP Tamsulosin Hydrochloride RS to a 250-mL volumetric flask, add 25 mL of methanol, and sonicate until dissolved. Dilute with Buffer stage medium to volume.
Buffer stage standard solution:
Dilute the Buffer stage standard stock solution with Buffer stage medium to obtain a final concentration of about 0.8 µg/mL.
Sample solution:
Pass a portion of the solution under test through a suitable filter.
Chromatographic system
Mode:
LC
Detector:
UV 230 nm
Analytical column:
3.9-mm × 15-cm; 5-µm packing L1
Guard column:
3-mm × 4-cm; packing L1
Column temperature:
35
Flow rate:
1.5 mL/min
Injection size:
200 µL
System suitability
Samples:
Acid stage standard solution and Buffer stage standard solution
Suitability requirements
Column efficiency:
NLT 1600, Acid stage standard solution; NLT 1300, Buffer stage standard solution
Relative standard deviation:
NMT 3.0%, Acid stage standard solution; NMT 1.5%, Buffer stage standard solution
Calculate the concentration of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at each time point (Ci).
At 2 h:
C1 = (rU/rS) × CS
At 3 h:
C2 = (rU/rS) × CS
At 8 h:
C3 = (rU/rS) × CS
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of the Standard solution (mg/mL) |
Calculate the cumulative percentage of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at each time point (Qi):
| V1 | = | = volume of Acid stage medium, 500 mL |
| VS | = | = volume of sample taken (mL) |
| Cn | = | = concentration of tamsulosin hydrochloride at each time point |
| V | = | = volume of Buffer stage medium, 1000 mL |
| L | = | = label claim (mg/Capsule) |
Tolerances:
See Table 3.
Table 3
| Time (h) |
Amount Dissolved |
|---|---|
| 2 | 0%–10% |
| 3 | 45%–68% |
| 8 | NLT 80% |
The percentages of the labeled amount of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at the times specified in the Buffer stage conform to Acceptance Table 2 in Dissolution  711
711 .
.
Test 5:
If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 5.
Acid stage medium:
0.003% polysorbate 80, pH 1.2 (proceed as directed for Test 2); 500 mL
Buffer stage medium:
Phosphate buffer, pH 7.2 (proceed as directed for Test 1); 500 mL
Apparatus 2:
50 rpm, with sinkers
Times:
2 h for the Acid stage medium, and 3 and 5 h for the Buffer stage medium (including the 2 h in the Acid stage medium)
Buffer solution:
Dissolve 1.0 g of octanesulfonic acid sodium salt and 1.4 g of monobasic potassium phosphate in 1 L of water. Adjust with potassium hydroxide to a pH of 6.5 ± 0.05.
Mobile phase:
Buffer solution and acetonitrile (3:2)
Standard stock solution:
0.04 mg/mL of USP Tamsulosin Hydrochloride RS in Buffer stage medium
Standard solution:
Dilute the Standard stock solution with Buffer stage medium to obtain a final concentration of 0.8 µg/mL.
Sample solution:
Centrifuge a portion of the solution under test at NMT 3000 rpm for NLT 20 min.
Analysis:
Perform the test using Acid stage medium. At 2 h after the start of the test, withdraw a sample of the solution under test. Carefully discard the Acid stage medium, replace it with the Buffer stage medium previously warmed, and continue the test. At 1 and 3 h after the replacement of the Medium, withdraw a sample of the solution under test. Replace the volume of Medium withdrawn with the same volume of Buffer stage medium, previously warmed.
Chromatographic system
Mode:
LC
Detector:
UV 225 nm
Column:
3.9-mm × 7.5-cm; 5-µm packing L7
Column temperature:
30
Flow rate:
1.5 mL/min
Injection size:
100 µL
System suitability
Sample:
Standard solution
Suitability requirements
Relative standard deviation:
NMT 4.0%
Calculate the concentration of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at each time point (Ci).
At 2 h:
C1 = (rU/rS) × CS
At 3 h:
C2 = (rU/rS) × CS
At 5 h:
C3 = (rU/rS) × CS
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of the Standard solution (mg/mL) |
Calculate the cumulative percentage of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at each time point (Qi):
| V1 | = | = volume of Acid stage medium, 500 mL |
| VS | = | = volume of sample taken (mL) |
| Cn | = | = concentration of tamsulosin hydrochloride at each time point |
| V | = | = volume of Buffer stage medium, 500 mL |
| L | = | = label claim (mg/Capsule) |
Tolerances:
See Table 4.
Table 4
| Time (h) |
Amount Dissolved |
|---|---|
| 2 | 15%–35% |
| 3 | 60%–80% |
| 5 | NLT 80% |
The percentages of the labeled amount of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at the times specified conform to Acceptance Table 2 in Dissolution  711
711 .
.
Test 6:
If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 6.
Acid stage medium:
0.003% polysorbate 80, pH 1.2 (proceed as directed for Test 2); 500 mL
Buffer stage medium:
Phosphate buffer, pH 7.2 (proceed as directed for Test 1); 500 mL
Apparatus 2:
100 rpm using a 40-mesh basket as a sinker, and the paddle height adjusted at 4.5 cm from the bottom of the vessel
Times:
2 h for the Acid stage medium, and 3 and 8 h for the Buffer stage medium (including the 2 h in the Acid stage medium)
Analysis:
Perform the test using Acid stage medium. At 2 h after the start of the test, withdraw a sample of the solution under test. Carefully discard the Acid stage medium, replace it with the Buffer stage medium previously warmed, and continue the test. At 1 h and 6 h after the replacement of the Medium, withdraw a sample of the solution under test. Replace the volume of Medium withdrawn with the same volume of Buffer stage medium, previously warmed.
Buffer solution:
Dissolve 3 g of sodium hydroxide and 8.7 mL of perchloric acid in 1900 mL of water, adjust with 0.5 M sodium hydroxide to a pH of 2.0 ± 0.05, and dilute with water to 2000 mL.
Mobile phase:
Buffer solution and acetonitrile (7:3)
Standard stock solution:
0.5 mg/mL of USP Tamsulosin Hydrochloride RS in methanol
Acid stage standard solution:
Dilute the Standard stock solution with Acid stage medium to obtain a final concentration of 0.8 µg/mL.
Buffer stage standard solution:
Dilute the Standard stock solution with Buffer stage medium to obtain a final concentration of 0.8 µg/mL.
Sample solution:
Pass a portion of the solution under test through a suitable filter of 0.45-µm pore size.
Chromatographic system
Mode:
LC
Detector:
UV 225 nm
Column:
4.6-mm × 15-cm, 3-µm packing L1
Column temperature:
40
Flow rate:
1.5 mL/min
Injection size:
100 µL
System suitability
Samples:
Acid stage standard solution and Buffer stage standard solution
Suitability requirements
Column efficiency:
NLT 7000
Tailing factor:
NMT 2.0
Relative standard deviation:
NMT 2.0%
Calculate the concentration of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at each time point (Ci).
At 2 h:
C1 = (rU/rS) × CS
At 3 h:
C2 = (rU/rS) × CS
At 8 h:
C3 = (rU/rS) × CS
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of the Standard solution (mg/mL) |
Calculate the cumulative percentage of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at each time point (Qi):
| V1 | = | = volume of Acid stage medium, 500 mL |
| VS | = | = volume of sample taken (mL) |
| Cn | = | = concentration of tamsulosin hydrochloride at each time point |
| V | = | = volume of Buffer stage medium, 500 mL |
| L | = | = label claim (mg/Capsule) |
Tolerances:
See Table 5.
Table 5
| Time (h) |
Amount Dissolved |
|---|---|
| 2 | 0%–20% |
| 3 | 47%–68% |
| 8 | NLT 80% |
The percentages of the labeled amount of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at the times specified conform to Acceptance Table 2 in Dissolution  711
711 .
.
Test 7:
If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 7.
Acid stage medium:
0.003% polysorbate 80, pH 1.2 (proceed as directed for Test 2); 500 mL
Buffer stage medium:
Phosphate buffer, pH 7.2 (proceed as directed for Test 1); 500 mL, deaerated
Apparatus 2:
100 rpm, with wire helix sinkers
Times:
2 h in the Acid stage medium, and 4 and 12 h in the Buffer stage medium (including the 2 h in the Acid stage medium)
Standard stock solution:
1 mg/mL of USP Tamsulosin Hydrochloride RS in alcohol
Acid stage standard solution:
Dilute the Standard stock solution with Acid stage medium to obtain a final concentration of about 0.2 µg/mL.
Buffer stage standard solution:
Dilute the Standard stock solution with Buffer stage medium to obtain a final concentration of about 0.8 µg/mL.
Sample solution:
Pass a portion of the solution under test through a suitable filter of 0.45-µm pore size.
Analysis:
Perform the test using Acid stage medium. At 2 h after the start of the test, withdraw a sample of the solution under test. Carefully discard the Acid stage medium, replace it with the Buffer stage medium previously warmed, and continue the test. At 1 and 6 h after the replacement of the Medium, withdraw a sample of the solution under test. Replace the volume of Medium withdrawn with the same volume of Buffer stage medium, previously warmed.
Mobile phase A:
2.76 g/L of monobasic potassium phosphate in water. Adjust with phosphoric acid to a pH of 2.5 ± 0.05.
Mobile phase B:
Acetonitrile
Gradient program:
See Table 6.
Table 6
| Time (min) |
Mobile phase A (%) |
Mobile phase B (%) |
|---|---|---|
| 0 | 86 | 14 |
| 3 | 86 | 14 |
| 4.5 | 30 | 70 |
| 5 | 86 | 14 |
Chromatographic system
Mode:
LC
Detector:
UV 225 nm
Column:
2-mm × 5-cm; packing L1
Column temperature:
30
Flow rate:
1.5 mL/min
Injection size:
100 µL
System suitability
Samples:
Acid stage standard solution and Buffer stage standard solution
Suitability requirements
Tailing factor:
NMT 1.5
Relative standard deviation:
NMT 2.0%
Calculate the concentration of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at each time point (Ci).
At 2 h:
C1 = (rU/rS) × CS
At 4 h:
C2 = (rU/rS) × CS
At 12 h:
C3 = (rU/rS) × CS
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of the Standard solution (mg/mL) |
Calculate the cumulative percentage of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at each time point (Qi):
| V1 | = | = volume of Acid stage medium, 500 mL |
| VS | = | = volume of sample taken (mL) |
| Cn | = | = concentration of tamsulosin hydrochloride at each time point |
| V | = | = volume of Buffer stage medium, 500 mL |
| L | = | = label claim (mg/Capsule) |
Tolerances:
See Table 7.
Table 7
| Time (h) |
Amount Dissolved |
|---|---|
| 2 | 5%–25% |
| 4 | 46%–66% |
| 12 | NLT 80% |
The percentages of the labeled amount of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at the times specified conform to Acceptance Table 2 in Dissolution  711
711 .
.
Test 8:
If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 8.
Acid stage medium:
0.003% polysorbate 80, pH 1.2 (proceed as directed for Test 2); 500 mL
Buffer stage medium:
Phosphate buffer, pH 7.2 (proceed as directed for Test 1); 500 mL
Apparatus 2:
100 rpm, with sinkers
Times:
2 h in the Acid stage medium, and 3 and 8 h in the Buffer stage medium (including the 2 h in the Acid stage medium)
Standard stock solution:
0.4 mg/mL of USP Tamsulosin Hydrochloride RS in methanol
Acid stage standard solution:
Dilute the Standard stock solution with Acid stage medium to obtain a final concentration of 0.8 µg/mL.
Buffer stage standard solution:
Dilute the Standard stock solution with Buffer stage medium to obtain a final concentration of 0.8 µg/mL.
Analysis:
Perform the test using Acid stage medium. At 2 h after the start of the test, withdraw a sample of the solution under test. Carefully discard the Acid stage medium, replace it with the Buffer stage medium previously warmed, and continue the test. At 1 and 6 h after the replacement of the Medium, withdraw a sample of the solution under test. Replace the volume of Medium withdrawn with the same volume of Buffer stage medium, previously warmed.
Sample solution:
Pass a portion of the solution under test through a suitable filter of 0.45-µm pore size.
Buffer solution:
Dissolve 6.8 g of dibasic ammonium phosphate in 800 mL of water, and add 2 mL of triethylamine. Adjust with phosphoric acid to a pH of 6.5. Dilute with water to 1000 mL.
Mobile phase:
Buffer solution and acetonitrile (7:3)
Chromatographic system
Mode:
LC
Detector:
UV 225 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Column temperature:
30
Flow rate:
1.0 mL/min
Injection size:
100 µL
Suitability requirements
Sample:
Buffer stage standard solution
Column efficiency:
NLT 2000 theoretical plates
Tailing factor:
NMT 2.0
Relative standard deviation:
NMT 2.0%
Calculate the concentration of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at each time point (Ci).
At 2 h:
C1 = (rU/rS) × CS
At 3 h:
C2 = (rU/rS) × CS
At 8 h:
C3 = (rU/rS) × CS
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of the Standard solution (mg/mL) |
Calculate the cumulative percentage of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at each time point (Qi):
| V1 | = | = volume of Acid stage medium, 500 mL |
| VS | = | = volume of sample taken (mL) |
| Cn | = | = concentration of tamsulosin hydrochloride at each time point |
| V | = | = volume of Buffer stage medium, 500 mL |
| L | = | = label claim (mg/Capsule) |
Tolerances:
See Table 8.
Table 8
| Time (h) |
Amount Dissolved |
|---|---|
| 2 | 0%–10% |
| 3 | 50%–70% |
| 8 | NLT 80% |
The percentages of the labeled amount of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at the times specified conform to Acceptance Table 2 in Dissolution  711
711 .
.
Test 9
If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 9.
Acid stage medium:
0.003% polysorbate 80, pH 1.2 (proceed as directed for Test 2); 500 mL
Buffer stage medium:
Phosphate buffer, pH 7.2 (proceed as directed for Test 1); 500 mL
Apparatus 2:
100 rpm, with helix wire coil sinker
Times:
2 h in Acid stage medium, and 3 and 8 h in Buffer stage medium (including the 2 h in Acid stage medium)
Standard stock solution:
0.05 mg/mL of USP Tamsulosin Hydrochloride RS in methanol
Acid stage standard solution:
Dilute the Standard stock solution with Acid stage medium to obtain a final concentration of 0.8 µg/mL.
Buffer stage standard solution:
Dilute the Standard stock solution with Buffer stage medium to obtain a final concentration of 0.8 µg/mL.
Sample solution:
Pass a portion of the solution under test through a suitable filter of 0.45-µm pore size.
Analysis:
Perform the test using Acid stage medium. At 2 h after the start of the test, withdraw a sample of the solution under test. Carefully discard the Acid stage medium, replace it with the Buffer stage medium previously warmed, and continue the test. At 1 and 6 h after the replacement of the Medium, withdraw a sample of the solution under test. Replace the volume of Medium withdrawn with the same volume of Buffer stage medium, previously warmed.
Buffer:
3.45 g/L of monobasic ammonium phosphate in water. Adjust with triethylamine to a pH of 6.5 ± 0.05.
Mobile phase:
Buffer and acetonitrile (3:2). Add 1 g of sodium 1-pentanesulfonate per L of the mixture.
Chromatographic system
Mode:
LC
Detector:
UV 225 nm
Column:
4.6-mm × 25-cm; 5-µm packing L7
Flow rate:
1.0 mL/min
Injection size:
100 µL
System suitability
Sample:
Acid stage standard solution or Buffer stage standard solution
Suitability requirements
Column efficiency:
NLT 3000 theoretical plates
Tailing factor:
NMT 2.0
Relative standard deviation:
NMT 2.0%
Calculate the concentration of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at each time point (Ci).
At 2 h:
C1 = (rU/rS) × CS
At 3 h:
C2 = (rU/rS) × CS
At 8 h:
C3 = (rU/rS) × CS
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the appropriate Standard solution |
| CS | = | = concentration of the appropriate Standard solution (mg/mL) |
Calculate the cumulative percentage of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at each time point (Qi):
| V1 | = | = volume of Acid stage medium, 500 mL |
| VS | = | = volume of sample taken (mL) |
| Cn | = | = concentration of tamsulosin hydrochloride at each time point |
| V | = | = volume of Buffer stage medium, 500 mL |
| L | = | = label claim (mg/Capsule) |
Tolerances:
See Table 9.
Table 9
| Time (h) |
Amount Dissolved |
|---|---|
| 2 | 0%–20% |
| 3 | 45%–65% |
| 8 | NLT 80% |
The percentages of the labeled amount of tamsulosin hydrochloride (C20H28N2O5S·HCl) dissolved at the times specified conform to Acceptance Table 2 in Dissolution  711
711 .
.
• Uniformity of Dosage Units  905
905 :
Meet the requirements
:
Meet the requirements
Procedure for content uniformity
[Note—Use the following Procedure for content uniformity if Procedure 1 is used in the Assay. For all other formulations, proceed as directed in the test for Uniformity of Dosage Units  905
905 . ]
. ]
Solution A, Solution B, Mobile phase, and Standard stock solution:
Prepare as directed in the Assay, Procedure 1.
Internal standard solution:
0.16 mg/mL of propylparaben in a mixture of acetonitrile and water (3:7)
Standard stock solution 1:
Transfer 5.0 mL of the Standard stock solution to a 25-mL volumetric flask, and dilute with a mixture of acetonitrile and water (3:7) to volume.
Standard solution:
Transfer 4.0 mL of Standard stock solution 1 to a suitable container. Add 5.0 mL of the Internal standard solution, and add the Mobile phase to make 40 mL.
Sample solution:
Place the contents of 1 Capsule into a Teflon-lined, screw-capped centrifuge tube. Place approximately 100 glass balls with a diameter of about 5 mm into the tube, add 20 mL of 0.05 N sodium hydroxide, heat at 50 for 10 min, and shake well for 30 min. Add 15 mL of a mixture of acetonitrile and Solution A (2:1) to the solution, and shake well. Add 5.0 mL of the Internal standard solution, and shake well. Centrifuge at 1500 rpm for 10 min, and use the supernatant, passing it if necessary through a membrane filter of pore size 0.5 µm or smaller.
for 10 min, and shake well for 30 min. Add 15 mL of a mixture of acetonitrile and Solution A (2:1) to the solution, and shake well. Add 5.0 mL of the Internal standard solution, and shake well. Centrifuge at 1500 rpm for 10 min, and use the supernatant, passing it if necessary through a membrane filter of pore size 0.5 µm or smaller.
Chromatographic system:
Proceed as directed in the Assay, Procedure 1, except inject 25 µL.
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of the labeled amount of tamsulosin hydrochloride (C20H28N2O5S·HCl) in the Capsule taken:
Result = (RU/RS) × (CS × VS/L) × 100
| RU | = | = ratio of the peak areas for tamsulosin and the internal standard from the Sample solution |
| RS | = | = ratio of the peak areas for tamsulosin and the internal standard from the Standard solution |
| CS | = | = concentration of USP Tamsulosin Hydrochloride RS in Standard stock solution 1 (mg/mL) |
| VS | = | = volume of Standard stock solution 1 taken to prepare the Standard solution (mL) |
| L | = | = label claim (mg/Capsule) |
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers. Store at controlled room temperature.
• Labeling:
When more than one Dissolution test is given, the labeling states the Dissolution test used only if Test 1 is not used.
• USP Reference Standards  11
11
USP Tamsulosin Hydrochloride RS
( )-(R)-5-[2-[[2-(o-Ethoxyphenoxy)ethyl]amino]propyl]-2-methoxybenzenesulfonamide monohydrochloride.
)-(R)-5-[2-[[2-(o-Ethoxyphenoxy)ethyl]amino]propyl]-2-methoxybenzenesulfonamide monohydrochloride.
C20H28N2O5S·HCI 444.97
(
C20H28N2O5S·HCI 444.97
1
A suitable sinker is available as catalog number CAPWHT-2S from www.qla-llc.com.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Elena Gonikberg, Ph.D.
Principal Scientific Liaison 1-301-816-8251 |
(SM32010) Monographs - Small Molecules 3 |
| Margareth R.C. Marques, Ph.D.
Senior Scientific Liaison 1-301-816-8106 |
(GCDF2010) General Chapters - Dosage Forms | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4746
Pharmacopeial Forum: Volume No. 34(5) Page 1193