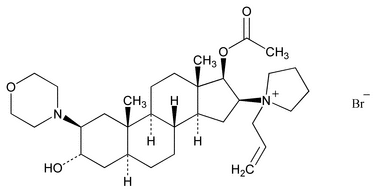
Rocuronium Bromide
C32H53BrN2O4 609.68
Pyrrolidinium, 1-[(2 ,3
,3 ,5
,5 ,16
,16 ,17
,17 )-17-(acetyloxy)-3-hydroxy-2-(4-morpholinyl)androstan-16-yl]-1-(2-propenyl)-, bromide;
)-17-(acetyloxy)-3-hydroxy-2-(4-morpholinyl)androstan-16-yl]-1-(2-propenyl)-, bromide;
1-Allyl-1-(3 ,17
,17 -dihydroxy-2
-dihydroxy-2 -morpholino-5
-morpholino-5 -androstan-16
-androstan-16 -yl)pyrrolidinium bromide, 17-acetate
-yl)pyrrolidinium bromide, 17-acetate


 [119302-91-9].
[119302-91-9].
(roe'' kure oh' nee um broe' mide).
C32H53BrN2O4 609.68
Pyrrolidinium, 1-[(2
1-Allyl-1-(3
DEFINITION
Rocuronium Bromide contains NLT 98.0% and NMT 102.0% of C32H53BrN2O4, calculated on the anhydrous and 2-propanol-free or acetic acid-free basis.
IDENTIFICATION
• B.
The retention time of the rocuronium bromide peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
• C. Identification Tests—General, Bromide  191
191 :
Meets the requirements of the silver nitrate test
:
Meets the requirements of the silver nitrate test
Sample solution:
10 mg/mL
ASSAY
• Procedure
Diluent:
Acetonitrile and water (9:1)
Buffer:
4.53 g/L of tetramethylammonium hydroxide pentahydrate. Adjust the solution with phosphoric acid to a pH of 7.4.
Mobile phase:
Acetonitrile and Buffer (9:1)
Standard solution:
1 mg/mL of USP Rocuronium Bromide RS in Diluent
Sample solution:
1 mg/mL of Rocuronium Bromide in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 210 nm
Column:
4.6-mm × 25-cm; 5-µm packing L3
Flow rate:
2 mL/min
Temperature:
30
Injection size:
5 µL
System suitability
[Note—The system may need equilibration for 4 h. ]
Sample:
Standard solution
Suitability requirements
Tailing factor:
NMT 2.0
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C32H53BrN2O4 in the portion of Rocuronium Bromide taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Rocuronium Bromide RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Rocuronium Bromide in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% on the anhydrous and 2-propanol-free or acetic acid-free basis
IMPURITIES
Organic Impurities
• Procedure
Diluent, Mobile phase, and Chromatographic system:
Proceed as directed in the Assay.
Peak identification solution:
1 mg/mL of USP Rocuronium Peak Identification Mixture RS in Diluent
Standard solution:
0.01 mg/mL of USP Rocuronium Bromide RS in Diluent
Sample solution:
10 mg/mL of Rocuronium Bromide in Diluent
Run time:
2.5 times the retention time for rocuronium
System suitability
[Note—The system may need equilibration for 4 h. ]
Sample:
Peak identification solution
Suitability requirements
Peak-to-valley ratio:
The ratio of the height of the rocuronium related compound H peak to the height of the valley between the rocuronium related compound H peak and the rocuronium peak is NLT 1.5.
Resolution:
NLT 3.5 between rocuronium and rocuronium related compound C
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Rocuronium Bromide taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
| rU | = | = peak response of any impurity from the Sample solution |
| rS | = | = peak response of rocuronium bromide from the Standard solution |
| CS | = | = concentration of USP Rocuronium Bromide RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Rocuronium Bromide in the Sample solution (mg/mL) |
| F | = | = relative response factor from Impurity Table 1 |
Acceptance criteria
Individual impurities:
See Impurity Table 1.
Total impurities:
NMT 1.5%
[Note—Disregard any peak eluting before rocuronium bromide related compound A, and any peak with an area less than 0.5 times that of the principal peak from the Standard solution. ]
Impurity Table 1
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Rocuronium related compound Aa | 0.20 | 2.1 | 0.2 |
| Rocuronium related compound Gb | 0.44 | 2.3 | 0.1 |
| Rocuronium related compound Fc | 0.75 | 0.79 | 0.1 |
| Rocuronium related compound Bd | 0.80 | 1.0 | 0.3 |
| Rocuronium related compound De | 0.90 | 1.0 | 0.1 |
| Rocuronium related compound Hf | 0.95 | 2.9 | 0.1 |
| Rocuronium bromide | 1.0 | — | — |
| Rocuronium related compound Cg | 1.20 | 1.0 | 0.3 |
| Rocuronium related compound Eh | 1.53 | 1.0 | 0.1 |
| Any individual unspecified impurity | — | — | 0.10 |
|
a
3
b
2
c
1-[3
d
1-[3
e
1-[3
f
1-[17
g
1-[3
h
1-[17
|
|||
SPECIFIC TESTS
• Limit of 2-Propanol
[Note—Perform this test only if 2-propanol is a known organic manufacturing process impurity. ]
Standard stock solution:
Transfer 35.0 µL of ethyl ether, 32.0 µL of 2-propanol, and 19.0 µL of methylene chloride to a 100-mL volumetric flask containing 90 mL of dimethylformamide (DMF), and dilute with DMF to volume.
Standard solution:
Transfer 2.5 mL of the Standard stock solution to a 25-mL volumetric flask containing 20 mL of DMF, and dilute with DMF to volume.
Dilute standard solution:
Transfer 1.0 mL of the Standard solution and 4.0 mL of water to a 20-mL headspace vial. Immediately close the vial with a cap, and mix.
Sample solution:
Transfer 50 mg of Rocuronium Bromide to a 20-mL headspace vial. Dissolve in 1.0 mL of DMF. Add 4 mL of water, immediately close the vial with a cap, and mix.
Chromatographic system
Mode:
GC
Detector:
Flame ionization
Column:
0.32-mm × 60-cm fused silica column coated with a 1.8-µm layer of liquid phase G43
Temperature:
See the temperature program table below.
| Initial Temperature ( |
Temperature Ramp ( |
Final Temperature ( |
Hold Time at Final Temperature (min) |
|---|---|---|---|
| 50 | 0 | 50 | 8 |
| 50 | 20 | 250 | 8 |
Injector:
140
Detector block:
280
Carrier gas:
Helium with a linear velocity of 55 cm/s or nitrogen with a linear velocity of 25 cm/s
Split ratio:
1:6
Head space autosampler
Sample equilibration temperature:
90
Sample equilibration time:
15 min
Transfer line temperature:
140
System suitability
Sample:
Dilute standard solution
[Note—The relative retention times for ethyl ether, 2-propanol, and methylene chloride are 0.87, 1.0, and 1.08, respectively. ]
Suitability requirements
Resolution:
NLT 1.0 between ethyl ether and 2-propanol; NLT 1.0 between 2-propanol and methylene chloride
Relative standard deviation:
NMT 10.0% for the 2-propanol peak
Analysis
Samples:
Dilute standard solution and Sample solution
Calculate the percentage of 2-propanol in the portion of Rocuronium Bromide taken:
Result = [(rU/rS) × (V × D/W) × 100]/F
| rU | = | = peak response of any impurity from the Sample solution |
| rS | = | = peak response of rocuronium bromide from the Dilute standard solution |
| V | = | = volume of 2-propanol taken to prepare the Standard stock solution (µL) |
| D | = | = relative density of 2-propanol (mg/µL), 0.786 |
| W | = | = weight of Rocuronium Bromide taken to prepare the Sample solution (mg) |
| F | = | = dilution factor for the Standard solution, 1000 |
Acceptance criteria:
NMT 1.0%
• Limit of Acetic Acid
[Note—Perform this test only if acetic acid is a known organic manufacturing process impurity. ]
Mobile phase:
6.1 g of sodium perchlorate in 800 mL of water. Adjust with 1 N sulfuric acid to a pH of 2.0. Dilute to 1 L.
Standard solution:
0.2 mg/mL of glacial acetic acid in Mobile phase
Sample solution:
6.0 mg/mL of Rocuronium Bromide in Mobile phase. [Note—Sonication may be necessary to completely dissolve the rocuronium bromide. ]
Chromatographic system
Mode:
LC
Detector:
UV 205 nm
Column:
4.6-mm × 15-cm; packing L1
Temperature:
30
Flow rate:
1 mL/min
Injection size:
20 µL
System suitability
Sample:
Standard solution
[Note—The relative retention time of acetic acid is about 3.8 min. ]
Suitability requirements
Column efficiency:
NLT 5000 theoretical plates
Tailing factor:
NMT 1.8
Relative standard deviation:
NMT 5.0% for three injections
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of acetic acid in the portion of Rocuronium Bromide taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response for acetic acid from the Sample solution |
| rS | = | = peak response for acetic acid from the Standard solution |
| CS | = | = concentration of acetic acid in the Standard solution (mg/mL) |
| CU | = | = concentration of Rocuronium Bromide in the Sample solution (mg/mL) |
Acceptance criteria:
NMT 5.0%
• Water Determination, Method Ic  921
921 :
NMT 4.0%
:
NMT 4.0%
• Optical Rotation, Specific Rotation  781
781 :
Between 28.5
:
Between 28.5 and 32.0
and 32.0 , measured on the anhydrous and solvent-free basis at 20
, measured on the anhydrous and solvent-free basis at 20
Sample solution:
10 mg/mL in 0.05 M hydrochloric acid
• Color and Achromicity  631
631
Reference solution:
Mix 33 mL of Matching Fluid G and 67 mL of water.
Sample solution:
10 mg/mL of Rocuronium Bromide in water
Analysis:
Proceed as directed for Color and Achromicity  631
631 .
.
Acceptance criteria:
The Sample solution is not more intensely colored than the Reference solution.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers, protected from light and moisture. Store at  20
20 or below. If the article contains acetic acid, store it between 2
or below. If the article contains acetic acid, store it between 2 and 8
and 8 .
.
• USP Reference Standards  11
11
USP Rocuronium Bromide RS
USP Rocuronium Peak Identification Mixture RS
Mixture of approximately 0.2% to 0.4% each of rocuronium related compound A, rocuronium related compound B, rocuronium related compound C, rocuronium related compound D, rocuronium related compound E, rocuronium related compound F, rocuronium related compound G, and rocuronium related compound H in a matrix of rocuronium bromide.
Mixture of approximately 0.2% to 0.4% each of rocuronium related compound A, rocuronium related compound B, rocuronium related compound C, rocuronium related compound D, rocuronium related compound E, rocuronium related compound F, rocuronium related compound G, and rocuronium related compound H in a matrix of rocuronium bromide.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Hariram Ramanathan, M.S.
Associate Scientific Liaison 1-301-816-8313 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4584
Pharmacopeial Forum: Volume No. 34(3) Page 648