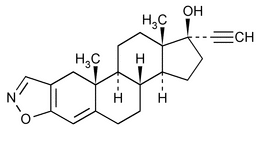
Danazol
(dan' a zol).
Pregna-2,4-dien-20-yno[2,3-d]isoxazol-17-ol, (17
17
» Danazol contains not less than 97.0 percent and not more than 102.0 percent of C22H27NO2, calculated on the dried basis.
Packaging and storage—
Preserve in tight, light-resistant containers.
Identification—
A:
Infrared Absorption  197K
197K .
.
B:
Ultraviolet Absorption  197U
197U —
—
Solution:
prepared as directed in the Assay.
Loss on drying  731
731 —
Dry it at a pressure not exceeding 5 mm of mercury at 60
—
Dry it at a pressure not exceeding 5 mm of mercury at 60 to constant weight: it loses not more than 2.0% of its weight.
to constant weight: it loses not more than 2.0% of its weight.
Chromatographic purity—
Solvent—
Prepare a mixture of chloroform and methanol (9:1).
Standard solutions—
Dissolve an accurately weighed quantity of USP Danazol RS in Solvent to obtain a solution having a known concentration of 1 mg per mL. Dilute quantitatively with Solvent to obtain Standard solutions having the following compositions:
| Standard
solution |
Dilution | Concentration (µg RS per mL) |
Percentage (%, for comparison with test specimen) |
|---|---|---|---|
| A | (1 in 2) | 500 | 1.0 |
| B | (1 in 4) | 250 | 0.5 |
| C | (1 in 10) | 100 | 0.2 |
| D | (1 in 20) | 50 | 0.1 |
Test solution—
Dissolve an accurately weighed quantity of Danazol in Solvent to obtain a solution containing 50 mg per mL.
Procedure—
Apply separately 5 µL of the Test solution and 5 µL of each Standard solution to a suitable thin-layer chromatographic plate (see Chromatography  621
621 ) coated with a 0.25-mm layer of chromatographic silica gel mixture. Position the plate in a chromatographic chamber and develop the chromatograms in a solvent system consisting of a mixture of cyclohexane and ethyl acetate (7:3) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and allow the solvent to evaporate in warm, circulating air. Examine the plate under short-wavelength UV light. Expose the plate to iodine vapors for 5 minutes. Compare the intensities of any secondary spots observed in the chromatogram of the Test solution with those of the principal spots in the chromatograms of the Standard solutions: the sum of the intensities of secondary spots obtained from the Test solution corresponds to not more than 1.0% of related compounds, with no single impurity corresponding to more than 0.5%.
) coated with a 0.25-mm layer of chromatographic silica gel mixture. Position the plate in a chromatographic chamber and develop the chromatograms in a solvent system consisting of a mixture of cyclohexane and ethyl acetate (7:3) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and allow the solvent to evaporate in warm, circulating air. Examine the plate under short-wavelength UV light. Expose the plate to iodine vapors for 5 minutes. Compare the intensities of any secondary spots observed in the chromatogram of the Test solution with those of the principal spots in the chromatograms of the Standard solutions: the sum of the intensities of secondary spots obtained from the Test solution corresponds to not more than 1.0% of related compounds, with no single impurity corresponding to more than 0.5%.
Assay—
Dissolve about 100 mg of Danazol, accurately weighed and previously dried, in about 50 mL of alcohol in a 100-mL volumetric flask, swirl until dissolved, dilute with alcohol to volume, and mix. Transfer 2.0 mL of this solution to a 100-mL volumetric flask, dilute with alcohol to volume, and mix. Similarly, dissolve an accurately weighed quantity of USP Danazol RS in alcohol to obtain a Standard solution having a known concentration of about 20 µg per mL. Concomitantly determine the absorbances of both solutions in 1-cm cells at the wavelength of maximum absorbance at about 285 nm, using alcohol as the blank. Calculate the quantity, in mg, of C22H27NO2 in the portion of Danazol taken by the formula:
5C(AU / AS)
in which C is the concentration, in µg per mL, of USP Danazol RS in the Standard solution; and AU and AS are the absorbances of the solution of Danazol and the Standard solution, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Domenick Vicchio, Ph.D.
Senior Scientific Liaison 1-301-998-6828 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2804
Pharmacopeial Forum: Volume No. 27(6) Page 3269