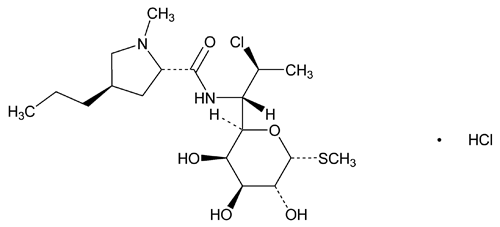
Clindamycin Hydrochloride
C18H33ClN2O5S·HCl 461.45
C18H33ClN2O5S·HCl·H2O 479.47
l-threo- -d-galacto-Octopyranoside, methyl 7-chloro-6,7,8-trideoxy-6-[[(1-methyl-4-propyl-2-pyrrolidinyl)-carbonyl]amino]-1-thio-, (2S-trans)-, monohydrochloride;
-d-galacto-Octopyranoside, methyl 7-chloro-6,7,8-trideoxy-6-[[(1-methyl-4-propyl-2-pyrrolidinyl)-carbonyl]amino]-1-thio-, (2S-trans)-, monohydrochloride;
Methyl 7-chloro-6,7,8-trideoxy-6-(1-methyl-trans-4-propyl-l-2-pyrrolidinecarboxamido)-1-thio-l-threo- -d-galacto-octopyranoside monohydrochloride
-d-galacto-octopyranoside monohydrochloride


 [21462-39-5].
[21462-39-5].
Monohydrate

 [58207-19-5].
[58207-19-5].
 A.
A. USP35 Infrared Absorption
USP35 Infrared Absorption  197M
197M
 • B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay. USP35
USP35
(klin'' da mye' sin hye'' droe klor' ide).
C18H33ClN2O5S·HCl 461.45
C18H33ClN2O5S·HCl·H2O 479.47
l-threo-
Methyl 7-chloro-6,7,8-trideoxy-6-(1-methyl-trans-4-propyl-l-2-pyrrolidinecarboxamido)-1-thio-l-threo-
Monohydrate
DEFINITION
Clindamycin Hydrochloride is the hydrated hydrochloride salt of clindamycin, a substance produced by the chlorination of lincomycin. It has a potency equivalent to NLT 800 µg/mg of C18H33ClN2O5S.
IDENTIFICATION
Change to read:
•
Add the following:
ASSAY
Change to read:
• Procedure
Buffer:
6.8 g/L of monobasic potassium phosphate in water. Adjust with 8 N potassium hydroxide to a pH of 7.5.
Mobile phase:
Acetonitrile and Buffer (9:11)

 USP35
USP35
Standard solution:
1 mg/mL of USP Clindamycin Hydrochloride RS in Mobile phase
Sample solution:
1 mg/mL of Clindamycin Hydrochloride in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 210 nm
Column:
4.6-mm × 25-cm; 5-µm packing L1
Flow rate:
1 mL/min
Injection size:
10 µL
System suitability
Sample:
Standard solution
Suitability requirements
 [Note—USP Clindamycin Hydrochloride RS contains clindamycin B and 7-epiclindamycin as minor components. ]
[Note—USP Clindamycin Hydrochloride RS contains clindamycin B and 7-epiclindamycin as minor components. ]
 USP35
USP35

 USP35
USP35
Resolution:
NLT 2.4 between clindamycin B and 7-epiclindamycin and NLT 3.0 between 7-epiclindamycin and clindamycin
Tailing factor:
NMT 1.2 for the clindamycin peak
Relative standard deviation:
NMT 1.0% for the clindamycin peak
Analysis
Samples:
Standard solution and Sample solution
Record the chromatograms for a period of time that is twice the retention time of the clindamycin peak.
Calculate the potency of C18H33ClN2O5S, in µg/mg, in the portion of Clindamycin Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × P
| rU | = | = peak area from the Sample solution |
| rS | = | = peak area from the Standard solution |
| CS | = | = concentration of USP Clindamycin Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Clindamycin Hydrochloride in the Sample solution (mg/mL) |
| P | = | = potency of clindamycin in USP Clindamycin Hydrochloride RS (µg/mg) |
Acceptance criteria:
NLT 800 µg/mg
IMPURITIES
Organic Impurities
• Procedure
Buffer and Mobile phase:
Prepare as directed in the Assay.
Standard stock solution:
0.5 mg/mL of USP Lincomycin Hydrochloride RS and 1 mg/mL of USP Clindamycin Hydrochloride RS in Mobile phase
Standard solution:
50 µg/mL of USP Lincomycin Hydrochloride RS and 100 µg/mL of USP Clindamycin Hydrochloride RS from Standard stock solution in Mobile phase
Sample solution:
5 mg/mL of Clindamycin Hydrochloride in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 210 nm
Column:
4.6-mm × 25-cm; 5-µm packing L1
Flow rate:
1 mL/min
Injection size:
10 µL
Analysis
Samples:
Standard solution and Sample solution
Record the chromatograms for a period of time that is six times the retention time of clindamycin.
Calculate the percentage of lincomycin in the portion of Clindamycin Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × P × F × 100
| rU | = | = peak response of lincomycin from the Sample solution |
| rS | = | = peak response of lincomycin from the Standard solution |
| CS | = | = concentration of USP Lincomycin Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Clindamycin Hydrochloride in the Sample solution (mg/mL) |
| P | = | = potency of USP Lincomycin RS (µg/mg) |
| F | = | = conversion factor, 0.001 mg/µg |
Calculate the percentage of all other related compounds in the portion of Clindamycin Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × P × F × 100
| rU | = | = peak response of each individual related compound, other than lincomycin, from the Sample solution |
| rS | = | = peak response of clindamycin from the Standard solution |
| CS | = | = concentration of USP Clindamycin Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = concentration of the Sample solution (mg/mL) |
| P | = | = potency of USP Clindamycin Hydrochloride RS (µg/mg) |
| F | = | = conversion factor, 0.001 mg/µg |
Acceptance criteria:
See Table 1.
Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Lincomycina | 0.4 | — |
| Clindamycin B | 0.65 | 2.0 |
| 7-Epiclindamycin | 0.8 | 4.0 |
| Clindamycin | 1.0 | — |
| Any other individual related compound |
— | 1.0 |
| Total related compoundsb |
— | 6.0 |
|
a
Lincomycin is controlled in the total of all related compounds. There is no individual acceptance criterion for this compound.
b
Total of all related compounds including lincomycin.
|
||
SPECIFIC TESTS
• Crystallinity  695
695 :
Meets the requirements
:
Meets the requirements
• pH  791
791 :
3.0–5.5, in a 100-mg/mL solution
:
3.0–5.5, in a 100-mg/mL solution
• Water Determination, Method I  921
921 :
3.0%–6.0%
:
3.0%–6.0%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers.
• USP Reference Standards  11
11
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ahalya Wise, M.S.
Senior Scientific Liaison 1-301-816-8161 |
(SM12010) Monographs - Small Molecules 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2702
Pharmacopeial Forum: Volume No. 36(6) Page 1520