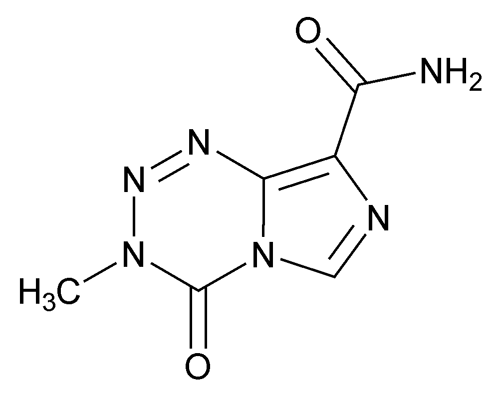
Add the following:
(tem'' oh zoe' loe mide).
C6H6N6O2 194.15
Imidazo[5,1-d]-1,2,3,5-tetrazine-8-carboxamide, 3,4-dihydro-3-methyl-4-oxo-;
3,4-Dihydro-3-methyl-4-oxoimidazo[5,1-d]-as-tetrazine-8-carboxamide
DEFINITION
Temozolomide contains NLT 98.0% and NMT 102.0% of C6H6N6O2, calculated on the as-is basis.
[Caution—Temozolomide is cytotoxic. Great care should be taken to prevent inhaling particles of Temozolomide and exposure to the skin.
]
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
[Note—Shake the solutions containing temozolomide to aid the dissolution. Do not sonicate. ]
• Procedure
Solution A:
0.5% (v/v) of glacial acetic acid in water
Mobile phase:
Solution A and methanol (96:4), containing 0.94 g/L of sodium 1-hexanesulfonate (0.005 M)
Diluent:
Dimethylsulfoxide. [Note—Use a freshly opened bottle. ]
Standard solution:
1.0 mg/mL of USP Temozolomide RS in Diluent
Sample solution:
1.0 mg/mL of Temozolomide in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 270 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Flow rate:
1 mL/min
Injection size:
10 µL
System suitability
Sample:
Standard solution
Suitability requirements
Relative standard deviation:
NMT 1.5%
Tailing factor:
NMT 1.9
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C6H6N6O2 in the portion of Temozolomide taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak area from the Sample solution |
| rS | = | = peak area from the Standard solution |
| CS | = | = concentration of USP Temozolomide RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Temozolomide in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% calculated on the as-is basis
IMPURITIES
• Residue on Ignition  281
281 :
NMT 0.1%
:
NMT 0.1%
• Heavy Metals, Method II  231
231 :
NMT 30 ppm
:
NMT 30 ppm
• Organic Impurities
[Note—Shake the solutions containing temozolomide to aid the dissolution. Do not sonicate. ]
Mobile phase, Diluent, and Sample solution:
Proceed as directed in the Assay.
Standard solution:
2.0 µg/mL each of USP Temozolomide RS and USP Dacarbazine Related Compound A RS in Diluent
System suitability solution:
0.5 µg/mL each of USP Temozolomide RS and USP Dacarbazine Related Compound A RS in Diluent, from the Standard solution
Peak identification solution:
Mix 5 mL of 0.1 N hydrochloric acid and 5 mL of 1.0 mg/mL of USP Temozolomide RS in Diluent. Heat the container for 1 h on a steam or boiling water bath. [Note—The preparation forms 2-azahypoxanthine, temozolomide acid, and dacarbazine related compound A. ]
Chromatographic system:
Proceed as directed in the Assay, using a run time of NLT 3.2 times the retention time of the temozolomide peak.
System suitability
Samples:
Standard solution and System suitability solution
Suitability requirements
Resolution:
NLT 2.0 between the temozolomide and dacarbazine related compound A peaks, Standard solution
Relative standard deviation:
NMT 10% for both dacarbazine related compound A and temozolomide peaks, System suitability solution
Analysis
Samples:
Sample solution, Standard solution, and Peak identification solution
Inject the Peak identification solution and identify the organic impurities according to the relative retention times given in Table 1.
Calculate the percentage of dacarbazine related compound A (free base) in the portion of Temozolomide taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
| rU | = | = peak area of dacarbazine related compound A from the Sample solution |
| rS | = | = peak area of dacarbazine related compound A from the Standard solution |
| CS | = | = concentration of USP Dacarbazine Related Compound A RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Temozolomide in the Sample solution (mg/mL) |
| Mr1 | = | = molecular weight of dacarbazine related compound A (free base), 126.12 |
| Mr2 | = | = molecular weight of dacarbazine related compound A (hydrochloride salt), 162.58 |
Calculate the percentage of any other individual impurity in the portion of Temozolomide taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
| rU | = | = peak area of each impurity from the Sample solution |
| rS | = | = peak area of temozolomide from the Standard solution |
| CS | = | = concentration of USP Temozolomide RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Temozolomide in the Sample solution (mg/mL) |
| F | = | = relative response factor for each individual impurity (see Table 1) |
Acceptance criteria:
See Table 1. [Note—Disregard any unspecified impurity peaks less than 0.05%. ]
Table 1
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| 2-Azahypoxanthinea | 0.42 | 1.6 | 0.2 |
| Temozolomide related compound Ab | 0.53 | 1.0 | 0.5 |
| Temozolomide acidc | 0.84 | 1.0 | 0.1 |
| Temozolomide | 1.0 | — | — |
| Dacarbazine related compound A (free base)d | 1.37 | — | 0.1 |
| Any unspecified impurity | — | 1.0 | 0.10 |
| Total impurities | — | — | 0.8 |
|
a
4a,5-Dihydro-4H-imidazo[4,5-d][1,2,3]triazin-4-one.
b
4-Diazo-4H-imidazole-5-carboxamide.
c
3-Methyl-4-oxo-3,4-dihydroimidazo[5,1-d][1,2,3,5]tetrazine-8-carboxylic acid.
d
5-Aminoimidazole-4-carboxamide. It is a free base of dacarbazine related compound A.
|
|||
SPECIFIC TESTS
• Water Determination, Method Ic  921
921 :
NMT 0.4%
:
NMT 0.4%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers, and store at room temperature.
• USP Reference Standards  11
11
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Feiwen Mao, M.S.
Senior Scientific Liaison 1-301-816-8320 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4782
Pharmacopeial Forum: Volume No. 37(1)