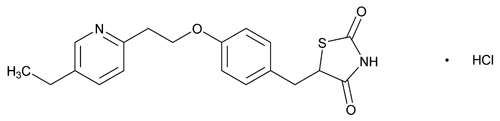
Pioglitazone Hydrochloride
C19H20N2O3S·HCl 392.90
2,4-Thiazolidinedione, 5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-, monohydrochloride, (±)-;
(±)-5-[p-[2-(5-Ethyl-2-pyridyl)ethoxy]benzyl]-2,4-thiazolidinedione monohydrochloride

 [112529-15-4].
[112529-15-4].
(pye'' oh gli' ta zone hye'' droe klor' ide).
C19H20N2O3S·HCl 392.90
2,4-Thiazolidinedione, 5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-, monohydrochloride, (±)-;
(±)-5-[p-[2-(5-Ethyl-2-pyridyl)ethoxy]benzyl]-2,4-thiazolidinedione monohydrochloride
DEFINITION
Pioglitazone Hydrochloride contains NLT 98.0% and NMT 102.0% of C19H20N2O3S·HCl, calculated on the anhydrous basis.
IDENTIFICATION
• B. Identification Tests–General, Chloride  191
191 :
Dissolve 25 mg of Pioglitazone Hydrochloride in 0.5 mL of nitric acid, and add 2 mL of dilute nitric acid. It meets the requirements of the test for Chloride.
:
Dissolve 25 mg of Pioglitazone Hydrochloride in 0.5 mL of nitric acid, and add 2 mL of dilute nitric acid. It meets the requirements of the test for Chloride.
• C.
The retention time of the pioglitazone peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
Mobile phase:
Acetonitrile, 0.1 M ammonium acetate, and glacial acetic acid (25:25:1)
Standard solution:
Prepare a 0.5 mg/mL solution of USP Pioglitazone Hydrochloride RS in methanol, and dilute with Mobile phase to obtain a solution containing 50 µg/mL of pioglitazone hydrochloride.
System suitability stock solution:
0.5 mg/mL of USP Pioglitazone Hydrochloride RS and 0.13 mg/mL of benzophenone in methanol
System suitability solution:
Dilute System suitability stock solution with Mobile phase to obtain a solution containing 50 µg/mL of pioglitazone hydrochloride and 13 µg/mL of benzophenone.
Sample solution:
Prepare a 0.5 mg/mL solution of pioglitazone hydrochloride in methanol, and dilute with Mobile phase to obtain a solution containing 50 µg/mL of pioglitazone hydrochloride.
Chromatographic system
Mode:
LC
Detector:
UV 269 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Column temperature:
25 ± 2.5
Flow rate:
0.7 mL/min
[Note—Adjust the flow rate so that the retention time of the pioglitazone peak is about 7 min. ]
Injection size:
20 µL
System suitability
Samples:
System suitability solution and Standard solution
[Note—The approximate relative retention times for pioglitazone and benzophenone are 1.0 and 2.6, respectively. ]
Suitability requirements
Tailing factor:
NMT 1.5 for pioglitazone and benzophenone, System suitability solution
Resolution:
NLT 15 between pioglitazone and benzophenone, System suitability solution
Relative standard deviation:
NMT 2.0% for six replicate injections, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C19H20N2O3S·HCl in the portion of Pioglitazone Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Pioglitazone Hydrochloride RS in the Standard solution (µg/mL) |
| CU | = | = concentration of Pioglitazone Hydrochloride in the Sample solution (µg/mL) |
Acceptance criteria:
98.0%–102.0% on the anhydrous basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.1%
:
NMT 0.1%
• Heavy Metals
Sodium sulfide solution:
5 g of sodium sulfide in 10 mL of water and 30 mL of glycerin
Magnesium nitrate solution:
100 mg/mL of magnesium nitrate in alcohol
Standard solution:
Place 10 mL of Magnesium nitrate solution in a platinum or porcelain crucible. Ignite the alcohol to burn. Cool, add 1 mL of sulfuric acid, heat carefully, and ignite at 550 ± 50 . Cool and add 3 mL of hydrobromic acid. Proceed as directed from this point under Sample solution, adding 1.0 mL of Standard Lead Solution (see Heavy Metals
. Cool and add 3 mL of hydrobromic acid. Proceed as directed from this point under Sample solution, adding 1.0 mL of Standard Lead Solution (see Heavy Metals  231
231 , Special Reagents) before adding water to make 50 mL.
, Special Reagents) before adding water to make 50 mL.
Sample solution:
Place 1.0 g of pioglitazone hydrochloride in a platinum or porcelain crucible. Mix with 10 mL of Magnesium nitrate solution. Ignite the alcohol to burn, and carbonize by gradual heating. Cool, add 1 mL of sulfuric acid, heat carefully, and incinerate by ignition at 550 ± 50 . If carbonized substances remain, moisten with a small amount of sulfuric acid, and incinerate by ignition. Cool, dissolve the residue in 3 mL of hydrobromic acid, and evaporate on a water bath to dryness. Wet the residue with 3 drops of hydrochloric acid, add 10 mL of water and dissolve by warming. Add 1 drop of phenolphthalein TS, and add ammonia TS dropwise until a pale red color develops. Add 2 mL of 1 N acetic acid, filter if necessary, wash with 10 mL of water, transfer the filtrate and washings to a Nessler tube, and add water to make 50 mL.
. If carbonized substances remain, moisten with a small amount of sulfuric acid, and incinerate by ignition. Cool, dissolve the residue in 3 mL of hydrobromic acid, and evaporate on a water bath to dryness. Wet the residue with 3 drops of hydrochloric acid, add 10 mL of water and dissolve by warming. Add 1 drop of phenolphthalein TS, and add ammonia TS dropwise until a pale red color develops. Add 2 mL of 1 N acetic acid, filter if necessary, wash with 10 mL of water, transfer the filtrate and washings to a Nessler tube, and add water to make 50 mL.
Analysis:
Add 1 drop of Sodium sulfide solution to each of the tubes containing the Standard solution and Sample solution. Mix thoroughly and allow to stand for 5 min. Compare the colors of both solutions by viewing the tubes downward or transversely against a white background. The Sample solution has no more color than the Standard solution.
Acceptance criteria:
NMT 10 ppm
Organic Impurities
• Procedure
Mobile phase and System suitability stock solution:
Proceed as directed in the Assay.
System suitability solution:
Dilute the System suitability stock solution with Mobile phase to obtain a solution containing 25 µg/mL of pioglitazone hydrochloride and 6.5 µg/mL of benzophenone.
Sample solution:
0.2 mg/mL of pioglitazone hydrochloride dissolved in 20% of the final volume with methanol, then diluted with Mobile phase to final volume
Standard solution:
1 µg/mL of pioglitazone hydrochloride prepared by diluting the Sample solution with Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 269 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Column temperature:
25 ± 2.5
Flow rate:
0.7 mL/min
[Note—Adjust the flow rate so that the retention time of the pioglitazone peak is about 7 min. ]
Injection size:
40 µL
Run time:
At least four times the retention time of pioglitazone
System suitability
Samples:
System suitability solution and Standard solution
Suitability requirements
Tailing factor:
NMT 1.5 for pioglitazone and benzophenone, System suitability solution
Resolution:
NLT 15 between pioglitazone and benzophenone, System suitability solution
Relative standard deviation:
NMT 3.0%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Pioglitazone Hydrochloride taken:
Result = (rU/rS) × D × 100
| rU | = | = peak response of each individual impurity from the Sample solution |
| rS | = | = peak response of pioglitazone from the Standard solution |
| D | = | = dilution factor used to prepare the Standard solution, 0.005 |
Acceptance criteria
Individual impurities:
See Impurity Table 1.
Total impurities:
NMT 0.5%
Impurity Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|
|---|---|---|---|
| Hydroxypioglitazonea | 0.7 | 0.15 | |
| Pioglitazone | 1.0 | — | |
| Didehydropioglitazoneb | 1.4 | 0.15 | |
| N-Alkylpioglitazonec | 3.0 | 0.15 | |
| Any other individual impurity | — | 0.10 | |
|
a
(±)-5-{4-[2-(5-Ethylpyridin-2-yl)ethoxy]benzyl}-5-hydroxythiazolidine-2,4-dione.
b
(Z)-5-{4-[2-(5-Ethylpyridin-2-yl)ethoxy]benzylidene}thiazolidine-2,4-dione.
c
(±)-5-{4-[2-(5-Ethylpyridin-2-yl)ethoxy]benzyl}-3-[2-(5-ethylpyridin-2-yl)ethyl]thiazolidine-2,4-dione.
|
|||
SPECIFIC TESTS
• Water Determination, Method Ic  921
921 :
NMT 0.5%
:
NMT 0.5%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers, and store at room temperature.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Elena Gonikberg, Ph.D.
Principal Scientific Liaison 1-301-816-8251 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4329
Pharmacopeial Forum: Volume No. 36(1) Page 126