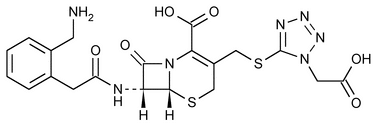
Ceforanide
(sef or' a nide).
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[[2-(aminomethyl)phenyl]acetyl]amino]-3-[[[1-(carboxymethyl)1H-tetrazol-5-yl]thio]methyl]-8-oxo-, (6R-trans)-.
(6R,7R)-7-[2-(
7-[o-(Aminomethyl)phenylacetamido]-3-[[[1-(carboxymethyl)-1H-tetrazol-5-yl]thio]methyl]-3-cephem-4-carboxylic acid
» Ceforanide contains not less than 900 µg and not more than 1050 µg of ceforanide (C20H21N7O6S2) per mg.
Packaging and storage—
Preserve in tight containers.
Labeling—
Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
Identification—
B:
The retention time of the major peak for ceforanide in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
Bacterial endotoxins  85
85 —
Where the label states that Ceforanide is sterile or must be subjected to further processing during the preparation of injectable dosage forms, it contains not more than 0.25 USP Endotoxin Unit per mg of ceforanide.
—
Where the label states that Ceforanide is sterile or must be subjected to further processing during the preparation of injectable dosage forms, it contains not more than 0.25 USP Endotoxin Unit per mg of ceforanide.
Sterility  71
71 —
Where the label states that Ceforanide is sterile, it meets the requirements when tested as directed for Membrane Filtration under Test for Sterility of the Product to be Examined, except to dissolve 6 g of Ceforanide in Fluid A to each 1000 mL of which has been added 10 g of sterile l-lysine, and to rinse the membrane with three 100-mL portions of Fluid D and one 100-mL portion of Fluid A.
—
Where the label states that Ceforanide is sterile, it meets the requirements when tested as directed for Membrane Filtration under Test for Sterility of the Product to be Examined, except to dissolve 6 g of Ceforanide in Fluid A to each 1000 mL of which has been added 10 g of sterile l-lysine, and to rinse the membrane with three 100-mL portions of Fluid D and one 100-mL portion of Fluid A.
pH  791
791 :
between 2.5 and 4.5, in a suspension containing 50 mg per mL.
:
between 2.5 and 4.5, in a suspension containing 50 mg per mL.
Water, Method I  921
921 :
not more than 5.0%.
:
not more than 5.0%.
Assay—
Mobile phase—
Mix 18 mL of tetrabutylammonium hydroxide solution (1 in 10) and 8.6 mL of 11 N potassium hydroxide, and add the mixture to 700 mL of water. Add 200 mL of methanol, adjust with phosphoric acid to a pH of 7.0, and add water to obtain 1000 mL of solution, making adjustments if necessary (see System Suitability under Chromatography  621
621 ). Filter, using a filter having a porosity of 1 µm or finer, and degas.
). Filter, using a filter having a porosity of 1 µm or finer, and degas.
Standard preparation—
Dissolve an accurately weighed quantity of USP Ceforanide RS in Mobile phase to obtain a solution having a known concentration of about 1 mg per mL. Use this solution within 5 minutes.
Assay preparation—
Using a suitable quantity of Ceforanide, accurately weighed, proceed as directed under Standard preparation. Use this solution within 5 minutes.
Chromatographic system
(see Chromatography  621
621 )—The liquid chromatograph is equipped with a 254-nm detector and a 4-mm × 30-cm column that contains 5- to 10-µm packing L1. The flow rate is about 1 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed under Procedure: the column efficiency determined from the analyte peak is not less than 1900 theoretical plates; the tailing factor for the analyte peak is not more than 1.2; the capacity factor, k¢, is not less than 1.8 and not more than 5.0; and the relative standard deviation for replicate injections is not more than 1.5%.
)—The liquid chromatograph is equipped with a 254-nm detector and a 4-mm × 30-cm column that contains 5- to 10-µm packing L1. The flow rate is about 1 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed under Procedure: the column efficiency determined from the analyte peak is not less than 1900 theoretical plates; the tailing factor for the analyte peak is not more than 1.2; the capacity factor, k¢, is not less than 1.8 and not more than 5.0; and the relative standard deviation for replicate injections is not more than 1.5%.
Procedure—
Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in µg, of C20H21N7O6S2 in each mg of the Ceforanide taken by the formula:
(CP / M)(rU / rS)
in which C is the concentration, in mg per mL, of USP Ceforanide RS in the Standard preparation; P is the potency, in µg per mg, of the USP Ceforanide RS; M is the concentration, in mg per mL, of the Assay preparation, based on the amount of Ceforanide taken and the extent of dilution; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ahalya Wise, M.S.
Senior Scientific Liaison 1-301-816-8161 |
(SM12010) Monographs - Small Molecules 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology |
USP35–NF30 Page 2555