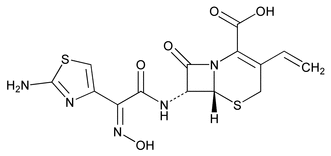
Cefdinir
C14H13N5O5S2 395.41
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[(2-amino-4-thiazolyl)(hydroxyimino)acetyl]amino]-3-ethenyl-8-oxo-, [6R-[6 ,7
,7 (Z)]]-;
(Z)]]-;
( )-(6R,7R)-7-[2-(2-Amino-4-thiazolyl)glyoxylamido]-8-oxo-3-vinyl-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 72-(Z)-oxime
)-(6R,7R)-7-[2-(2-Amino-4-thiazolyl)glyoxylamido]-8-oxo-3-vinyl-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 72-(Z)-oxime


 [91832-40-5].
[91832-40-5].
 Acceptance criteria:
See Table 2.
Acceptance criteria:
See Table 2.
 USP35
USP35
(sef' di nir).
C14H13N5O5S2 395.41
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[(2-amino-4-thiazolyl)(hydroxyimino)acetyl]amino]-3-ethenyl-8-oxo-, [6R-[6
(
DEFINITION
Cefdinir contains NLT 940 µg/mg and NMT 1030 µg/mg of cefdinir (C14H13N5O5S2), calculated on the anhydrous basis.
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
Solution A:
14.2 g/L of anhydrous dibasic sodium phosphate
Solution B:
13.6 g/L of monobasic potassium phosphate
Solution C:
Dilute tetramethylammonium hydroxide (10%) with water to obtain a 0.1% solution. Adjust with 10% phosphoric acid to a pH of 5.5.
Solution D:
37.2 mg/mL of edetate disodium
Buffer:
Combine appropriate amounts of Solution A and Solution B (about 2:1) to obtain a solution with a pH of 7.0.
Mobile phase:
Acetonitrile, methanol, Solution C, and Solution D (300:200:4500:2)
System suitability solution:
0.2 mg/mL of USP Cefdinir RS and 0.5 mg/mL of USP Cefdinir Related Compound A RS in Buffer
Standard solution:
0.2 mg/mL of USP Cefdinir RS in Buffer
Sample solution:
0.2 mg/mL of Cefdinir in Buffer
Chromatographic system
Mode:
LC
Detector:
UV 254 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Column temperature:
40
Flow rate:
1 mL/min
Injection size:
5 µL
System suitability
Samples:
System suitability solution and Standard solution. USP Cefdinir Related Compound A RS should produce four peaks.
Tailing factor:
NMT 1.5 for cefdinir, System suitability solution
Resolution:
NLT 1.2 between the second peak of cefdinir related compound A and cefdinir, System suitability solution
Relative standard deviation:
NMT 1.0%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the quantity, in µg/mg, of cefdinir (C14H13N5O5S2) in the portion of Cefdinir taken:
Result = (rU/rS) × (CS/CU) × P
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of the Standard solution (mg/mL) |
| CU | = | = concentration of the Sample solution (mg/mL) |
| P | = | = purity of USP Cefdinir RS (µg/mg) |
Acceptance criteria:
940–1030 µg/mg on the anhydrous basis
IMPURITIES
• Residue on Ignition  281
281 :
NMT 0.20%
:
NMT 0.20%
• Heavy Metals, Method II  231
231 :
10 ppm
:
10 ppm
Change to read:
• Organic Impurities
Solution A, Solution B, Solution C, Solution D, and Buffer:
Prepare as directed in the Assay.
Solution E:
To 1000 mL of Solution C add 0.4 mL of Solution D.
Solution F:
Acetonitrile, methanol, Solution C, and Solution D (300: 200: 500: 0.4)
Mobile phase:
See Table 1.
Table 1
| Time (min) |
Solution E (%) |
Solution F (%) |
|---|---|---|
| 0 | 95 | 5 |
| 2 | 95 | 5 |
| 22 | 75 | 25 |
| 32 | 50 | 50 |
| 37 | 50 | 50 |
| 38 | 95 | 5 |
| 58 | 95 | 5 |
System suitability solution 1:
15 µg/mL of cefdinir from the Sample solution, diluted with Solution C
System suitability solution 2:
1.5 µg/mL of cefdinir from System suitability solution 1, diluted with Solution C
System suitability solution 3:
 1.5 mg/mL of USP Cefdinir RS and 0.1 mg/mL of USP Cefdinir Related Compound A RS, dissolved initially in Buffer corresponding to 15% of the final volume, and diluted with Solution C to volume
1.5 mg/mL of USP Cefdinir RS and 0.1 mg/mL of USP Cefdinir Related Compound A RS, dissolved initially in Buffer corresponding to 15% of the final volume, and diluted with Solution C to volume USP35
USP35
Sample stock solution:
10 mg/mL of Cefdinir in Buffer
Sample solution:
1.5 mg/mL of cefdinir from the Sample stock solution, in Solution C. [Note—Prepare fresh immediately before use. ]
Chromatographic system
Mode:
LC
Detector:
UV 254 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Column temperature:
40
Flow rate:
1 mL/min
Injection size:
10 µL
System suitability
Samples:
System suitability solution 1, System suitability solution 2, and System suitability solution 3. USP Cefdinir Related Compound A RS should produce four peaks.
 USP35
USP35
Suitability requirements
 Resolution:
NLT 1.5 between cefdinir and the third peak of USP Cefdinir Related Compound A RS, System suitability solution 3
Resolution:
NLT 1.5 between cefdinir and the third peak of USP Cefdinir Related Compound A RS, System suitability solution 3 USP35
USP35
Response ratio:
The response of cefdinir from System suitability solution 2 is between 7% and 13% of that from System suitability solution 1.
Relative standard deviation:
NMT 2.0% for cefdinir, System suitability solution 3
Analysis
Sample:
Sample solution. Record the chromatogram for  at least 1.8 times the retention time of the cefdinir peak.
at least 1.8 times the retention time of the cefdinir peak. USP35
USP35
Calculate the percentage of each impurity in the portion of Cefdinir taken:
Result = (rU/rT) × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rT | = | = sum of all the peak responses from the Sample solution |
Table 2
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Thiazolylacetyl glycine oximea | 0.10 | 0.5 |
| Thiazolylacetyl glycine oxime acetalb | 0.12 | 0.5 |
| 3-Methyl cefdinirc | 0.74 | 0.7 |
| Cefdinir related compound A (cefdinir open ring lactone a)d,e | 0.85 | 0.7 |
| Cefdinir related compound A (cefdinir open ring lactone b)d,e | 0.93 | |
| Cefdinir related compound A (cefdinir open ring lactone c)d,e | 1.11 | |
| Cefdinir related compound A (cefdinir open ring lactone d)d,e | 1.14 | |
| Cefdinir lactonef | 1.22 | 0.5 |
| Cefdinir isoxazole analogg | 1.36 | 0.5 |
| E-Cefdinirh | 1.51 | 0.7 |
| Cefdinir decarboxy open ring lactone ai,j | 1.61 | 0.5 |
| Cefdinir decarboxy open ring lactone bi,j | 1.64 | |
| Any other individual, unidentified impurity | — | 0.2 |
| Total impurities | — | 3.0 |
|
a
1N-[(Z)-2-(2-Aminothiazol-4-yl)-2-(hydroxyimino)acetyl]glycine.
b
(Z)-2-(2-Aminothiazol-4-yl)-N-(2,2-dihydroxyethyl)-2-(hydroxyimino)acetamide.
c
(6R,7R)-7-[(Z)-2-(2-Aminothiazol-4-yl)-2-(hydroxyimino)acetamido]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid.
d
2(R)-2-[(Z)-2-(2-Aminothiazol-4-yl)-2-(hydroxyimino)acetamido)]-2-[(2RS,5RS)-5-methyl-7-oxo-2,4,5,7-tetrahydro-1H-furo[3,4-d][1,3]thiazin-2-yl]acetic acid.
e
Cefdinir related compound A is a mixture of 4 isomers labeled cefdinir open ring lactones a, b, c, and d. The sum of the values is reported. The limit for the sum of the 4 isomers is 0.7%.
f
(Z)-2-(2-Aminothiazol-4-yl)-2-(hydroxyimino)-N-{(3RS,5aR,6R)-3-methyl-1,7-dioxo-1,3,4,5a,6,7-hexahydroazeto[2,1-b]furo[3,4-d][1,3]thiazin-6-yl}acetamide.
g
(6R,7R)-7-(4-Hydroxyisoxazole-3-carboxamido)-8-oxo-3-vinyl-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid.
h
(6R,7R)-7-[(E)-2-(2-Aminothiazol-4-yl-)-2-(hydroxyimino)acetamido]-8-oxo-3-vinyl-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid.
i
(Z)-2-(2-Aminothiazol-4-yl)-2-(hydroxyimino)-N-{[(2RS,5RS)-5-methyl-7-oxo-2,4,5,7-tetrahydro-1H-furo[3,4-d][1,3]thiazin-2-yl]methyl}acetamide.
j
Cefdinir decarboxy open ring lactone is a mixture of 2 isomers labeled cefdinir decarboxy open ring lactones a and b. The sum of the values is reported. The limit for sum of the 2 isomers is 0.5%.
|
||
SPECIFIC TESTS
• Optical Rotation, Specific Rotation  781S
781S
Sample solution:
10 mg/mL in Buffer, as obtained in the Assay
Acceptance criteria:
 61
61 to
to  67
67 at 20
at 20
• Water Determination, Method I  921
921 :
NMT 2.0% for anhydrous; 4.0%–8.5% for hydrated forms. For this monograph, the term “hydrated forms” refers to several known forms of Cefdinir. Use a mixture of formamide and methanol (2:1) as the solvent.
:
NMT 2.0% for anhydrous; 4.0%–8.5% for hydrated forms. For this monograph, the term “hydrated forms” refers to several known forms of Cefdinir. Use a mixture of formamide and methanol (2:1) as the solvent.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers.
• USP Reference Standards  11
11
USP Cefdinir RS
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ahalya Wise, M.S.
Senior Scientific Liaison 1-301-816-8161 |
(SM12010) Monographs - Small Molecules 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2537
Pharmacopeial Forum: Volume No. 36(6) Page 1509