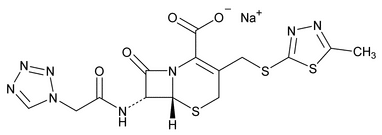
Cefazolin Sodium
C14H13N8NaO4S3 476.49
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-[[(5-methyl-1,3,4-thiadiazol-2-yl)thio]methyl]-8-oxo-7-[[(1H-tetrazol-1-yl)acetyl]amino]-, monosodium salt (6R-trans);
Monosodium (6R,7R)-3-[[(5-methyl-1,3,4-thiadiazol-2-yl)thio]methyl]-8-oxo-7-[2-(1H-tetrazol-1-yl)acetamido]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate

 [27164-46-1].
[27164-46-1].
(sef a' zoe lin soe' dee um).
C14H13N8NaO4S3 476.49
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-[[(5-methyl-1,3,4-thiadiazol-2-yl)thio]methyl]-8-oxo-7-[[(1H-tetrazol-1-yl)acetyl]amino]-, monosodium salt (6R-trans);
Monosodium (6R,7R)-3-[[(5-methyl-1,3,4-thiadiazol-2-yl)thio]methyl]-8-oxo-7-[2-(1H-tetrazol-1-yl)acetamido]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
DEFINITION
Cefazolin Sodium has a potency equivalent to NLT 89.1% and NMT 110.1% of cefazolin sodium (C14H13NaN8O4S3), calculated on the anhydrous basis.
IDENTIFICATION
• A. Ultraviolet Absorption  197U
197U
Sample solution:
20 µg/mL in 0.1 M sodium bicarbonate
Acceptance criteria:
Meets the requirements
• B.
The retention time of the major peak for cefazolin in the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
• C. Identification Tests—General, Sodium  191
191 :
Meets the requirements
:
Meets the requirements
ASSAY
• Procedure
Buffer A:
0.9 g/L of anhydrous dibasic sodium phosphate and 1.298 g/L of citric acid monohydrate in water
Buffer B:
5.68 g/L of anhydrous dibasic sodium phosphate and 3.63 g/L of monobasic potassium phosphate in water
Mobile phase:
Acetonitrile and Buffer A (1:9). Pass through a membrane filter having a 10-µm or finer pore size.
Internal standard solution:
7.5 mg/mL of salicylic acid in methanol and Buffer B (1:9). Dissolve first in methanol, using 10% of the final volume, and dilute with water to volume.
Standard stock solution:
1 mg/mL of USP Cefazolin RS in Buffer B
Standard solution:
50 µg/mL of cefazolin from the Standard stock solution and 0.4 mg/mL of salicylic acid from the Internal standard solution in Buffer B
Sample stock solution:
1 mg/mL of Cefazolin Sodium in Buffer B
Sample solution:
50 µg/mL of cefazolin sodium from the Sample stock solution and 0.4 mg/mL of salicylic acid from the Internal standard solution in Buffer B
Chromatographic system
Mode:
LC
Detector:
UV 254 nm
Column:
4.0-mm × 30-cm; 10-µm packing L1
Flow rate:
2 mL/min
Injection size:
10 µL
System suitability
Sample:
Standard solution
[Note—The relative retention times for salicylic acid and cefazolin are about 0.7 and 1.0, respectively. ]
Suitability requirements
Resolution:
NLT 4.0 between the analyte and the internal standard peaks
Column efficiency:
NLT 1500 theoretical plates
Tailing factor:
NMT 1.5
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of cefazolin sodium (C14H13N8NaO4S3) in the portion of Cefazolin Sodium taken:
Result = (RU/RS) × (CS/CU) × (Mr1/Mr2) × 100
| RU | = | = peak response ratio of cefazolin to the internal standard from the Sample solution |
| RS | = | = peak response ratio of cefazolin to the internal standard from the Standard solution |
| CS | = | = concentration of USP Cefazolin RS, calculated on the anhydrous basis, in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of Cefazolin Sodium in the Sample solution (mg/mL) |
| Mr1 | = | = molecular weight of cefazolin sodium, 476.49 |
| Mr2 | = | = molecular weight of cefazolin, 454.51 |
Acceptance criteria:
89.1%–110.1% on the anhydrous basis
IMPURITIES
• Organic Impurities
[Note—Use the Sample solution immediately after preparation. ]
Buffer A:
6.8 g/L of monobasic potassium phosphate
Solution B:
6.8 g/L of monobasic potassium phosphate adjusted with 10% sodium hydroxide to a pH of 6.8 before final dilution
Solution C:
Acetonitrile and Buffer A (1:1)
Mobile phase:
See Table 1.
Table 1
| Time (min) |
Solution B (%) |
Solution C (%) |
|---|---|---|
| 0 | 98 | 2 |
| 7 | 98 | 2 |
| 15 | 85 | 15 |
| 30 | 80 | 20 |
| 35 | 80 | 20 |
| 45 | 50 | 50 |
| 50 | 50 | 50 |
| 55 | 98 | 2 |
| 65 | 98 | 2 |
Blank:
Use Solution B.
System suitability stock solution:
2 mg/mL of USP Cefazolin RS in 0.05 M sodium hydroxide. Set the solution aside at room temperature for 5 min. [Note—The cefazolin epimer is formed upon treatment of cefazolin with sodium hydroxide. ]
System suitability solution:
System suitability stock solution and Buffer B (1:24)
Standard solution:
25 µg/mL of USP Cefazolin RS in Solution B
Sample solution:
2.5 mg/mL of Cefazolin Sodium in Solution B
Chromatographic system
Mode:
LC
Detector:
UV 210 and 254 nm
Column:
4.6-mm × 25-cm; 5-µm packing L1
Temperature:
30
Flow rate:
1.5 mL/min
Injection size:
20 µL
System suitability
Sample:
System suitability solution
Suitability requirements
Resolution:
NLT 8.0 between cefazolin and cefazolin epimer, 254 nm
Analysis
Samples:
Blank, Standard solution, and Sample solution
Calculate the percentage of tetrazolylacetic acid and tetrazolylacetamide acetal in the portion of Cefazolin Sodium taken:
Result = (rU(210)/rS(254)) × (CS/CU) × (1/F) × 100
| rU(210) | = | = peak response of tetrazolylacetic acid or tetrazolylacetamide acetal at 210 nm from the Sample solution |
| rS(254) | = | = peak response of cefazolin at 254 nm from the Standard solution |
| CS | = | = concentration of USP Cefazolin RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Cefazolin Sodium in the Sample solution (mg/mL) |
| F | = | = relative response factor (see Table 2) |
Calculate the percentage of each impurity other than tetrazolylacetic acid and tetrazolylacetamide acetal in the portion of Cefazolin Sodium taken:
Result = (rU(254)/rS(254)) × (CS/CU) × (1/F) × 100
| rU(254) | = | = peak response of each impurity other than tetrazolylacetic acid and tetrazolylacetamide acetal at 254 nm from the Sample solution |
| rS(254) | = | = peak response of cefazolin at 254 nm from the Standard solution |
| CS | = | = concentration of USP Cefazolin RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Cefazolin Sodium in the Sample solution (mg/mL) |
| F | = | = relative response factor (see Table 2) |
Acceptance criteria:
See Table 2. Disregard peaks corresponding to those in the Blank.
Table 2
| Name | Analytical Wavelength (nm) |
Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|---|
| Tetrazolylacetic acida | 210 | 0.07 | 0.40 | 1.0 |
| Tetrazolylacetamide acetalb | 210 | 0.08 | 0.33 | 1.0 |
| cCefazolin open-ring lactoned or Cefazolin 3-hydroxymethyle | 254 | 0.20 | 1.0 | 0.5 |
| Methylthiadiazole thiolf | 254 | 0.23 | 0.91 | 1.0 |
| 7-Aminocephalosporanic acidg | 254 | 0.42 | 1.1 | 1.0 |
| Cefazolin 3-methyl analogh | 254 | 0.44 | 0.87 | 1.0 |
| Cefazolin lactonei | 254 | 0.50 | 0.85 | 1.0 |
| Cefazolin acetoxy analogj | 254 | 0.61 | 0.68 | 1.0 |
| Cefazolin deacylatedk | 254 | 0.68 | 1.2 | 1.0 |
| Cefazoloic acid isomersl | 254 | 0.84 | 1.0 | 1.0 |
| Cefazolin | 254 | 1.0 | — | — |
| Cefazolin epimerm | 254 | 1.2 | 0.98 | 1.0 |
| Cefazolin pivaloyln | 254 | 1.4 | 0.92 | 1.0 |
| Any individual unspecified impurity | 254 | — | 1.0 | 0.1 |
| Total impurities | — | — | — | 3.5 |
|
a
2-(1H-Tetrazol-1-yl)acetic acid.
b
N-(2,2-Dihydroxyethyl)-2-(1H-tetrazol-1-yl)acetamide.
c
The identification of this impurity is tentative. The names of the most likely compounds are listed in footnotes d and e.
d
(R)-2-[2-(1H-Tetrazol-1-yl)acetamido]-2-[(R)-7-oxo-2,4,5,7-tetrahydro-1H-furo[3,4-d][1,3]thiazin-2-yl]acetic acid.
e
(6R,7R)-7-[2-(1H-Tetrazol-1-yl)acetamido]-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid.
f
5-Methyl-1,3,4-thiadiazole-2-thiol (MMTD).
g
(6R,7R)-3-(Acetoxymethyl)-7-amino-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (7-ACA).
h
(6R,7R)-7-[2-(1H-Tetrazol-1-yl)acetamido]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid.
i
N-{(5aR,6R)-1,7-Dioxo-1,3,4,5a,6,7-hexahydroazeto[2,1-b]furo[3,4-d][1,3]thiazin-6-yl}-2-(1H-tetrazol-1-yl)acetamide.
j
(6R,7R)-7-[2-(1H-Tetrazol-1-yl)acetamido]-3-(acetoxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid.
k
(6R,7R)-7-Amino-3-[(5-methyl-1,3,4-thiadiazol-2-ylthio)methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid.
l
Three isomers of this impurity may not be fully resolved by this method. The limit applies to the sum of the isomers, which are as follows:
Cefazolin open-ring delta-3: (2R)-2-{(R)-[2-(1H-Tetrazol-1-yl)acetamido](carboxy)methyl}-5-[(5-methyl-1,3,4-thiadiazol-2-ylthio)methyl]-3,6-dihydro-2H-1,3-thiazine-4-carboxylic acid. Cefazolin open-ring delta-2: (2R)-2-{(R)-[2-(1H-Tetrazol-1-yl)acetamido](carboxy)methyl}-5-[(5-methyl-1,3,4-thiadiazol-2-ylthio)methyl]-3,4-dihydro-2H-1,3-thiazine-4-carboxylic acid. Cefazolin open-ring delta-4: (2R)-2-{(R)-[2-(1H-Tetrazol-1-yl)acetamido](carboxy)methyl}-5-[(5-methyl-1,3,4-thiadiazol-2-ylthio)methyl]-5,6-dihydro-2H-1,3-thiazine-4-carboxylic acid.
m
(6R,7S)-7-[2-(1H-Tetrazol-1-yl)acetamido]-3-[(5-methyl-1,3,4-thiadiazol-2-ylthio)methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid.
n
(6R,7R)-3-((5-Methyl-1,3,4-thiadiazol-2-ylthio)methyl)-8-oxo-7-pivalamido-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid.
|
||||
SPECIFIC TESTS
• Optical Rotation, Specific Rotation  781S
781S
Sample solution:
55 mg/mL, in 0.1 M sodium bicarbonate
Acceptance criteria:
 10
10 to
to  24
24
• pH  791
791 :
4.0–6.0, in a solution containing 100 mg/mL of cefazolin
:
4.0–6.0, in a solution containing 100 mg/mL of cefazolin
• Water Determination, Method I  921
921 :
NMT 6.0%
:
NMT 6.0%
• Sterility Tests  71
71 :
Where the label states that Cefazolin Sodium is sterile, it meets the requirements when tested as directed for Test for Sterility of the Product to Be Examined, Membrane Filtration.
:
Where the label states that Cefazolin Sodium is sterile, it meets the requirements when tested as directed for Test for Sterility of the Product to Be Examined, Membrane Filtration.
• Bacterial Endotoxins Test  85
85 :
Where the label states that Cefazolin Sodium is sterile or must be subjected to further processing during the preparation of injectable dosage forms, it contains NMT 0.15 USP Endotoxin Unit/mg of cefazolin.
:
Where the label states that Cefazolin Sodium is sterile or must be subjected to further processing during the preparation of injectable dosage forms, it contains NMT 0.15 USP Endotoxin Unit/mg of cefazolin.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers.
• Labeling:
Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ahalya Wise, M.S.
Senior Scientific Liaison 1-301-816-8161 |
(SM12010) Monographs - Small Molecules 1 |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2533
Pharmacopeial Forum: Volume No. 34(6) Page 1438