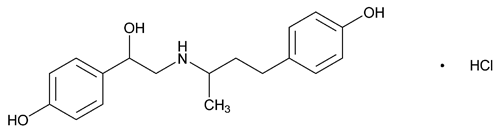
Ractopamine Hydrochloride Suspension
C18H23NO3·HCl 337.84
Benzenemethanol, 4-hydroxy- -[[[3-(4-hydroxyphenyl)-1-methylpropyl]amino]methyl]-, hydrochloride;
-[[[3-(4-hydroxyphenyl)-1-methylpropyl]amino]methyl]-, hydrochloride;
(±)-all-rac-p-Hydroxy- -[[[3-(p-hydroxyphenyl)-1-methylpropyl]amino]methyl]benzyl alcohol, hydrochloride
-[[[3-(p-hydroxyphenyl)-1-methylpropyl]amino]methyl]benzyl alcohol, hydrochloride


 [90274-24-1].
[90274-24-1].
C18H23NO3·HCl 337.84
Benzenemethanol, 4-hydroxy-
(±)-all-rac-p-Hydroxy-
DEFINITION
Ractopamine Hydrochloride Suspension contains NLT 10% and NMT 20%, by weight, of ractopamine hydrochloride (C18H23NO3·HCl) in water. [Note—The material partially precipitates out at room temperature to form a slurry, and redissolves when heated to 50 –60
–60 . ]
. ]
IDENTIFICATION
• Infrared Absorption  197K
197K
Sample:
Dry a portion of Ractopamine Hydrochloride Suspension under vacuum for 3 h at 60 .
.
ASSAY
• Procedure
Solution A:
5.75 mg/mL solution of monobasic ammonium phosphate adjusted with 10% phosphoric acid to a pH of 4.0 ± 0.1
Solution B:
1.1 mg/mL solution of 1-heptanesulfonic acid sodium salt in Solution A
Mobile phase:
Stabilizer-free tetrahydrofuran and Solution B (3:17)
Diluent:
Stabilizer-free tetrahydrofuran and water (3:17). [Note—The Standard solutions and System suitability solution are stable for up to 72 h at room temperature. The Sample solution is stable for up to 90 h at room temperature. ]
System suitability solution:
100 µg/mL of USP Ractopamine Hydrochloride RS and 10 µg/mL of USP Raspberry Alcohol RS in Diluent
Standard solution A:
0.08 mg/mL of USP Ractopamine Hydrochloride RS in Diluent
Standard solution B:
0.1 mg/mL of USP Ractopamine Hydrochloride RS in Diluent
Standard solution C:
0.12 mg/mL of USP Ractopamine Hydrochloride RS in Diluent
Sample stock solution:
Stir Ractopamine Hydrochloride Suspension in a 60 water bath for up to 1 h, to ensure complete dissolution. While hot, transfer 700 mg of the Ractopamine Hydrochloride Suspension dropwise to a 100-mL volumetric flask, and dilute with Diluent to volume.
water bath for up to 1 h, to ensure complete dissolution. While hot, transfer 700 mg of the Ractopamine Hydrochloride Suspension dropwise to a 100-mL volumetric flask, and dilute with Diluent to volume.
Sample solution:
Dilute a portion of the Sample stock solution with Diluent (1:10).
Chromatographic system
Mode:
LC
Detector:
UV 226 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Flow rate:
1 mL/min
Injection size:
20 µL
System suitability
Samples:
System suitability solution and Standard solution B
Suitability requirements
Resolution:
NLT 1.5 between raspberry alcohol and ractopamine, System suitability solution
Tailing factor:
NLT 0.7 and NMT 2.0 for the ractopamine peak, Standard solution B
Relative standard deviation:
NMT 2.0% for three replicate injections, Standard solution B
Analysis
Samples:
Standard solutions and Sample solution
Prepare a calibration curve using the three ractopamine peak responses from Standard solutions A, B, and C and their corresponding concentrations. From the graph determine the concentration, C, in mg/mL, of ractopamine hydrochloride in the Sample solution.
Calculate the percentage (w/w) of C18H23NO3·HCl in the portion of Ractopamine Hydrochloride Suspension taken:
Result = (V/W) × CS × D × 100
| V | = | = volume of the Sample stock solution, 100 mL |
| W | = | = weight of Ractopamine Hydrochloride Suspension taken (mg) |
| CS | = | = concentration of ractopamine hydrochloride from the Sample solution |
| D | = | = dilution factor to prepare the Sample solution, 10 |
Acceptance criteria:
10%–20% of C18H23NO3·HCl
IMPURITIES
Organic Impurities
• Procedure
Solution A:
5.75 mg/mL of monobasic ammonium phosphate in water; pH NLT 4.4
Solution B:
1.1 mg/mL of 1-heptanesulfonic acid sodium salt in Solution A
Solution C:
Acetonitrile and Solution B (1:9)
Solution D:
Acetonitrile and Solution B (17:33)
Mobile phase:
See the gradient table below.
| Time (min) |
Solution C (%) |
Solution D (%) |
|---|---|---|
| 0 | 100 | 0 |
| 22 | 0 | 100 |
| 32 | 0 | 100 |
| 37 | 100 | 0 |
| 55 | 100 | 0 |
Diluent:
Acetonitrile and water (1:4)
System suitability solution:
9 µg/mL each of USP Raspberry Ketone RS and USP Ractopamine Hydrochloride RS in Diluent
Blank:
Diluent
Sample solution A:
Stir Ractopamine Hydrochloride Suspension in a 60 water bath for up to 1 h, to ensure complete dissolution. While hot, transfer 200 mg of the Ractopamine Hydrochloride Suspension dropwise into a 50-mL volumetric flask, and dilute with Diluent to volume.
water bath for up to 1 h, to ensure complete dissolution. While hot, transfer 200 mg of the Ractopamine Hydrochloride Suspension dropwise into a 50-mL volumetric flask, and dilute with Diluent to volume.
Sample solution B:
Dilute a portion of Sample solution A with Diluent (1:100). [Note—The Sample solutions are stable for up to 48 h if stored at 5 . ]
. ]
Chromatographic system
Mode:
LC
Detector:
UV 226 nm
Column:
4.6-mm × 25-cm; 5-µm packing L1
Flow rate:
1 mL/min
Injection size:
20 µL
System suitability
Sample:
System suitability solution
Suitability requirements
Resolution:
NLT 2.0 between raspberry ketone and ractopamine
Analysis
Samples:
Blank, Sample solution A, and Sample solution B
[Note—Disregard any peaks that correspond to those in the Blank. Correct the response of the ractopamine peak in Sample solution B by subtracting the peak response at the retention time of ractopamine in the Blank. ]
Calculate the percentage of each individual impurity in the portion of Ractopamine Hydrochloride Suspension taken:
Result = (rA/rB) × 100/D
| rA | = | = peak response of each individual impurity from Sample solution A |
| rB | = | = corrected peak response for ractopamine from Sample solution B |
| D | = | = dilution factor to prepare Sample solution B, 100 |
Acceptance criteria
Individual impurities:
See Impurity Table 1.
Total impurities:
NMT 3.5%
Impurity Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Octopaminea | 0.37 | 0.5 |
| Tyramineb | 0.55 | 0.5 |
| N-Isopropyloctopaminec | 0.63 | 0.5 |
| Piperazinediphenold | 0.74 | 0.5 |
| Aminobutylphenole | 0.76 | 0.5 |
| Raspberry alcoholf | 0.85 | 0.5 |
| Raspberry ketoneg | 0.96 | 1.0 |
| Ractopamine | 1.0 | — |
| Deoxyractopamineh | 1.1 | 0.5 |
| Ractopamine O-methyli | 1.2 | 1.0 |
| Ractopamine N-hydroxybenzylj | 1.26 | 1.0 |
| Ractopamine cyclohexyl analogk | 1.29 | 0.5 |
| Ractopamine dimerl | 1.4 | 1.0 |
| Any individual unspecified impurity | — | 0.2 |
|
a
4-(2-Amino-1-hydroxyethyl)phenol.
b
4-(2-Aminoethyl)phenol.
c
4-[1-Hydroxy-2-(isopropylamino)ethyl]phenol.
d
4,4¢-(Piperazine-2, 5-diyl)diphenol.
e
4-(3-Aminobutyl)phenol.
f
4-(3-Hydroxybutyl)phenol.
g
4-(4-Hydroxyphenyl)butan-2-one.
h
4-[3-(4-Hydroxyphenethylamino)butyl]phenol.
i
4-{3-[2-(4-Hydroxyphenyl)-2-methoxyethylamino]butyl}phenol.
j
4-(1-Hydroxy-2-{(4-hydroxybenzyl)[4-(4-hydroxyphenyl)butan-2-yl]amino}ethyl)phenol.
k
4-{1-Hydroxy-2-[3-(4-hydoxyphenyl)-5-methylcyclohexylamino]ethyl}phenol).
l
4,4¢-(1,1¢-Oxybis{2-[4-(4-hydroxyphenyl)butan-2-ylamino]ethane-1,1-diyl})diphenol.
|
||
SPECIFIC TESTS
• Diastereomer Ratio
Solution A:
5.75 mg/mL of monobasic ammonium phosphate in water
Solution B:
Add 10 mL of triethylamine to 950 mL of Solution A, dilute with Solution A to 1000 mL, and adjust with phosphoric acid to a pH of 4.5.
Mobile phase:
Acetonitrile and Solution B (3:22)
Diluent:
Acetonitrile and Solution A (1:4)
System suitability solution:
0.4 mg/mL of USP Ractopamine Hydrochloride RS in Diluent
Sample solution:
Stir Ractopamine Hydrochloride Suspension in a 60 water bath for up to 1 h to ensure complete dissolution. While hot, transfer 275 mg of it dropwise into a 100-mL volumetric flask, and dilute with Diluent to volume.
water bath for up to 1 h to ensure complete dissolution. While hot, transfer 275 mg of it dropwise into a 100-mL volumetric flask, and dilute with Diluent to volume.
[Note—The Sample solution is stable for up to 36 h when stored at ambient conditions. ]
Chromatographic system
Mode:
LC
Detector:
UV 226 nm
Column:
4.6-mm × 25-cm; 5-µm packing L1
Flow rate:
1 mL/min
Injection size:
20 µL
System suitability
Sample:
System suitability solution
[Note—The elution order is RS,SR diastereoisomer followed by RR,SS diastereoisomer. ]
Suitability requirements
Resolution:
NLT 1.25 between the diastereomers
Analysis
Sample:
Sample solution
Calculate the RS,SR diastereomer content, in percentage:
Result = rA/(rA + rB) × 100
| rA | = | = peak response of the RS,SR diastereoisomer from the Sample solution |
| rB | = | = peak response of the RR,SS diastereoisomer from the Sample solution |
Acceptance criteria:
45%–49%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Store at a temperature not exceeding 70 .
.
• Labeling:
Label it to indicate that it is for veterinary use only.
• USP Reference Standards  11
11
USP Raspberry Alcohol RS
4-(3-Hydroxybutyl)phenol.
C10H14O2 166.22
4-(3-Hydroxybutyl)phenol.
C10H14O2 166.22
USP Raspberry Ketone RS
4-(4-Hydroxyphenyl)butan-2-one.
C10H12O2 164.20
4-(4-Hydroxyphenyl)butan-2-one.
C10H12O2 164.20
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Morgan Puderbaugh, B.S.
Associate Scientific Liaison 1-301-998-6833 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4513
Pharmacopeial Forum: Volume No. 35(5) Page 1171