Capreomycin Sulfate
Capreomycin sulfate

 [1405-37-4].
[1405-37-4].
C25H44N14O8 668.71
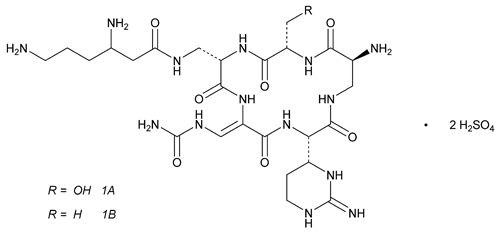
Capreomycin 1A (free base);
3,6-Diamino-N-({(2S,5S,11S,15S,Z)-15-amino-2-(hydroxymethyl)-11-[(R)-iminohexahydropyrimidin-4-yl]-3,6,9,12,16-pentaoxo-8-(ureidomethylene)-1,4,7,10,13-pentaazacyclohexadecan-5-yl}methyl)hexanamide

 [37290-35-6].
[37290-35-6].
C25H44N14O7 652.71
Capreomycin 1B (free base);
3,6-Diamino-N-({(2S,5S,11S,15S,Z)-15-amino-11-[(R)-iminohexahydropyrimidin-4-yl]-2-methyl-3,6,9,12,16-pentaoxo-8-(ureidomethylene)-1,4,7,10,13-pentaazacyclohexadecan-5-yl}methyl)hexanamide

 [33490-33-4].
[33490-33-4].
(kap'' ree oh mye' sin sul' fate).
Capreomycin sulfate
C25H44N14O8 668.71
Capreomycin 1A (free base);
3,6-Diamino-N-({(2S,5S,11S,15S,Z)-15-amino-2-(hydroxymethyl)-11-[(R)-iminohexahydropyrimidin-4-yl]-3,6,9,12,16-pentaoxo-8-(ureidomethylene)-1,4,7,10,13-pentaazacyclohexadecan-5-yl}methyl)hexanamide
C25H44N14O7 652.71
Capreomycin 1B (free base);
3,6-Diamino-N-({(2S,5S,11S,15S,Z)-15-amino-11-[(R)-iminohexahydropyrimidin-4-yl]-2-methyl-3,6,9,12,16-pentaoxo-8-(ureidomethylene)-1,4,7,10,13-pentaazacyclohexadecan-5-yl}methyl)hexanamide
DEFINITION
Capreomycin Sulfate is the disulfate salt of capreomycin, a polypeptide mixture produced by the growth of Streptomyces capreolus, suitable for parenteral use. It has a potency equivalent to NLT 700 µg/mg and NMT 1050 µg/mg of capreomycin.
IDENTIFICATION
• B.
The retention times of the major peaks of the Sample solution correspond to those of the System suitability solution, as obtained in the test for Capreomycin 1 Content.
ASSAY
• Procedure:
Proceed as directed in Antibiotics—Microbial Assays  81
81 .
.
Acceptance criteria:
700–1050 µg/mg
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 3.0%, the charred residue being moistened with 2 mL of nitric acid and 5 drops of sulfuric acid
:
NMT 3.0%, the charred residue being moistened with 2 mL of nitric acid and 5 drops of sulfuric acid
• Heavy Metals, Method II  231
231 :
NMT 30 ppm
:
NMT 30 ppm
SPECIFIC TESTS
• Capreomycin 1 Content
Solution A:
0.4 mg/mL of ammonium bisulfate in water. Pass through a filter of 0.5-µm or less pore size.
Mobile phase:
Methanol and Solution A (2:3)
System suitability solution:
0.25 mg/mL of USP Capreomycin Sulfate RS in water
Sample solution:
0.25 mg/mL of Capreomycin Sulfate in water
Chromatographic system
Mode:
LC
Detector:
UV 268 nm
Column:
4.6-mm × 25-cm; 5-µm packing L10
Column temperature:
30
Flow rate:
1.5 mL/min
Injection size:
20 µL
System suitability
Sample:
System suitability solution
[Note—The relative retention times of capreomycin 1A and capreomycin 1B are 0.85 and 1.0, respectively. ]
Suitability requirements
Resolution:
NLT 1.5 between capreomycin 1A and capreomycin 1B
Tailing factor:
NMT 3.5 for the major peaks (capreomycin 1A and capreomycin 1B)
Analysis
[Note—The chromatographic run time is at least five times the retention time of the capreomycin 1A peak. ]
Sample:
Sample solution
Calculate the percentage of capreomycin 1 in the portion of Capreomycin Sulfate taken:
Result = [(r1A + r1B)/rT] × 100
| r1A | = | = peak area response of capreomycin 1A |
| r1B | = | = peak area response of capreomycin 1B |
| rT | = | = total response for all peaks |
Acceptance criteria:
NLT 90.0%
• Loss on Drying  731
731 :
Dry 100 mg in a vacuum at a pressure not exceeding 5 mm of mercury at 100
:
Dry 100 mg in a vacuum at a pressure not exceeding 5 mm of mercury at 100 for 4 h: it loses NMT 10.0% of its weight.
for 4 h: it loses NMT 10.0% of its weight.
• Bacterial Endotoxins Test  85
85 :
Where it is intended for use in preparing injectable dosage forms: NMT 0.35 USP Endotoxin Unit/mg of capreomycin
:
Where it is intended for use in preparing injectable dosage forms: NMT 0.35 USP Endotoxin Unit/mg of capreomycin
• Other Requirements:
Where the label states that Capreomycin Sulfate is sterile, it meets the requirements under Injections  1
1 .
.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers.
• Labeling:
Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ahalya Wise, M.S.
Senior Scientific Liaison 1-301-816-8161 |
(SM12010) Monographs - Small Molecules 1 |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2473
Pharmacopeial Forum: Volume No. 35(6) Page 1448