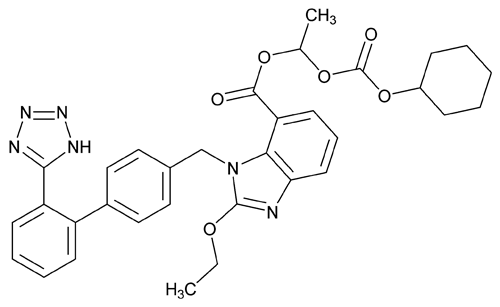
Add the following:
(kan'' de sar' tan sye lex' e til).
C33H34N6O6
1H-Benzimidazole-7-carboxylic acid, 2-ethoxy-1-[[2¢-(1H-tetrazol-5-yl)[1,1¢-biphenyl]-4-yl]methyl]-, 1-[[(cyclohexyloxy)carbonyl]oxy]ethyl ester, (±);
(±)-1-Hydroxyethyl 2-ethoxy-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]-7-benzimidazolecarboxylate, cyclohexyl carbonate (ester). 610.66
DEFINITION
Candesartan Cilexetil contains NLT 98.7% and NMT 101.0% of C33H34N6O6, calculated on anhydrous basis.
IDENTIFICATION
• A. Infrared Absorption  197K
197K :
If the spectra obtained show differences, proceed with the samples prepared as follows. Separately dissolve a quantity of USP Candesartan Cilexetil RS and Candesartan Cilexetil in alcohol. [Note—Heating the solution may be necessary for complete dissolution. ] Cool the solution in an ice bath, filter the crystals, and dry at 105
:
If the spectra obtained show differences, proceed with the samples prepared as follows. Separately dissolve a quantity of USP Candesartan Cilexetil RS and Candesartan Cilexetil in alcohol. [Note—Heating the solution may be necessary for complete dissolution. ] Cool the solution in an ice bath, filter the crystals, and dry at 105 .
.
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the procedure for Organic Impurities.
ASSAY
• Procedure
Sample solution:
8.33 mg/mL of Candesartan Cilexetil in glacial acetic acid
Analysis:
Titrate with 8 mL of 0.1 N perchloric acid VS using a blank determination under the same conditions. Each mL of the Titrant is equivalent to 61.07 mg of C33H34N6O6.
Acceptance criteria:
98.7%–101.0% on the anhydrous basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.1%, determined from a 1-g sample
:
NMT 0.1%, determined from a 1-g sample
Organic Impurities
• Procedure
Diluent:
Acetonitrile and water (3:2)
Solution A:
Acetonitrile, glacial acetic acid, and water (57:1:43)
Solution B:
Acetonitrile, glacial acetic acid, and water (90:1:10)
Mobile phase:
See the gradient table below.
[Note—Equilibration for about 10 min may be necessary between injections. ]
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 100 | 0 |
| 30 | 0 | 100 |
System suitability solution:
0.04 mg/mL of USP Candesartan Cilexetil RS and 0.125 mg/mL of acenaphthene in Diluent
Standard solution:
4 µg/mL of USP Candesartan Cilexetil RS in Diluent
Sample solution:
0.4 mg/mL of Candesartan Cilexetil in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 254 nm
Column:
3.9-mm × 15-cm; 4-µm packing L1
Flow rate:
0.8 mL/min
Injection size:
10 µL
System suitability
[Note—The Mobile phase used for testing system suitability is 100% Solution A in an isocratic mode. ]
Sample:
System suitability solution
Suitability requirements
Resolution:
NLT 5.0 between candesartan cilexetil and acenaphtene
Tailing factor:
NMT 1.5 for candesartan cilexetil
Relative standard deviation:
NMT 3.0% for the candesartan cilexetil peak
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of each individual impurity in the portion of Candesartan Cilexetil taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of each individual impurity from the Sample solution |
| rS | = | = peak response of candesartan cilexetil from the Standard solution |
| CS | = | = concentration of USP Candesartan Cilexetil RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Candesartan Cilexetil in the Sample solution (mg/mL) |
Acceptance criteria
Individual impurities:
See Table 1.
Total impurities:
NMT 0.6%. [Note—Calculate the total impurities from the sum of all impurity peaks greater than or equal to 0.05%. ]
Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Ethyl candesartana | 0.4 | 0.2 |
| Desethyl candesartan cilexetilb | 0.5 | 0.3 |
| Candesartan cilexetil | 1.0 | — |
| N2-Ethyl candesartan cilexetilc | 2.0 | 0.2 |
| Any other unknown impurity |
— | 0.10 |
|
a
Ethyl 1-{[2¢-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}-2-ethoxybenzimidazole-7-carboxylate.
b
±1-(Cyclohexyloxycarbonyloxy)ethyl 1-{[2¢-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}-2-oxobenzimidazole-7-carboxylate.
c
±1-(Cyclohexyloxycarbonyloxy)ethyl 2-ethoxy-1-{[2¢-(N-ethyl-tetrazol-5-yl) biphenyl-4-yl]methyl}benzimidazole-7-carboxylate.
|
||
SPECIFIC TESTS
• Water Determination, Method 1  921
921 :
NMT 0.3%
:
NMT 0.3%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers, and store at controlled temperature.
• USP Reference Standards  11
11
USP Candesartan Cilexetil RS 
1H-Benzimidazole-7-carboxylic acid, 2-ethoxy-1-[[2¢-(1H-tetrazol-5-yl)[1,1¢-biphenyl]-4-yl]methyl]-, 1-[[(cyclohexyloxy)carbonyl]oxy]ethyl ester, (±);
(±)-1-Hydroxyethyl 2-ethoxy-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]-7-benzimidazolecarboxylate, cyclohexyl carbonate (ester). 610.66

1H-Benzimidazole-7-carboxylic acid, 2-ethoxy-1-[[2¢-(1H-tetrazol-5-yl)[1,1¢-biphenyl]-4-yl]methyl]-, 1-[[(cyclohexyloxy)carbonyl]oxy]ethyl ester, (±);
(±)-1-Hydroxyethyl 2-ethoxy-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]-7-benzimidazolecarboxylate, cyclohexyl carbonate (ester). 610.66
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Sujatha Ramakrishna, Ph.D.
Senior Scientific Liaison 1-301-816-8349 |
(SM22010) Monographs - Small Molecules 2 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2468
Pharmacopeial Forum: Volume No. 36(6) Page 1506