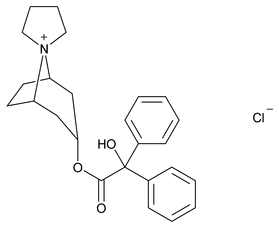
Trospium Chloride
C25H30ClNO3 427.96
Spiro [8-azoniabicyclo[3.2.1]octane-8,1¢-pyrrolidinium], 3-[(hydroxydiphenylacetyl)oxy]-, chloride, (1R,3r,5S);
(1R,3r,5S)-3-[(Hydroxydiphenylacetyl)oxy]spiro[8-azoniabicyclo[3.2.1]octane-8,1¢-pyrrolidinium] chloride

 [10405-02-4].
[10405-02-4].
(trose' pee um klor' ide).
C25H30ClNO3 427.96
Spiro [8-azoniabicyclo[3.2.1]octane-8,1¢-pyrrolidinium], 3-[(hydroxydiphenylacetyl)oxy]-, chloride, (1R,3r,5S);
(1R,3r,5S)-3-[(Hydroxydiphenylacetyl)oxy]spiro[8-azoniabicyclo[3.2.1]octane-8,1¢-pyrrolidinium] chloride
DEFINITION
Trospium Chloride contains NLT 98.0% and NMT 102.0% of C25H30ClNO3, calculated on the dried basis.
IDENTIFICATION
• B. Identification Tests—General, Chloride  191
191 :
Meets the requirements
:
Meets the requirements
ASSAY
• Procedure
Mobile phase:
Acetonitrile, triethylamine, phosphoric acid, and water (300:1:3:700)
System suitability solution:
0.01 mg/mL of USP Trospium Chloride RS, and 0.003 mg/mL each of USP Trospium Chloride Related Compound A RS and USP Trospium Chloride Related Compound B RS in Mobile phase
Standard solution:
0.6 mg/mL of USP Trospium Chloride RS in Mobile phase
Sample solution:
0.6 mg/mL of Trospium Chloride in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 215 nm
Column:
4.6-mm × 25-cm; 5-µm packing L1. (Alternatively, a 4.6-mm × 25-cm column that contains 5-µm packing L7 may be used.)
Column temperature:
40
Flow rate:
1 mL/min
Injection size:
10 µL
System suitability
Samples:
System suitability solution and Standard solution
Suitability requirements
Resolution:
NLT 3 between trospium chloride related compound B and trospium, System suitability solution
Relative standard deviation:
NMT 0.85% for six replicate injections, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C25H30ClNO3 in the portion of Trospium Chloride taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Trospium Chloride RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Trospium Chloride in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% on the dried basis
IMPURITIES
Inorganic Impurities
Organic Impurities
• Procedure 1
Mobile phase:
Proceed as directed in the Assay.
Standard solution:
0.01 mg/mL of USP Trospium Chloride RS, and 0.003 mg/mL each of USP Trospium Chloride Related Compound A RS and USP Trospium Chloride Related Compound B RS in Mobile phase
Sample solution:
3.0 mg/mL of Trospium Chloride in Mobile phase
Chromatographic system:
Proceed as directed in the Assay, except for the following parameters.
Injection size:
20 µL
Run time:
NLT 3 times the retention time of trospium
System suitability
Sample:
Standard solution
Suitability requirements
Resolution:
NLT 3 between trospium chloride related compound B and trospium
Analysis
Samples:
Standard solution and Sample solution
[Note—Reporting level for impurities is 0.05%. ]
Calculate the percentage of trospium chloride related compound A and trospium chloride related compound B in the portion of Trospium Chloride taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of trospium chloride related compound A or trospium chloride related compound B from the Sample solution |
| rS | = | = peak response of trospium chloride related compound A or trospium chloride related compound B from the Standard solution |
| CS | = | = concentration of trospium chloride related compound A or trospium chloride related compound B in the Standard solution (mg/mL) |
| CU | = | = concentration of Trospium Chloride in the Sample solution (mg/mL) |
Calculate the percentage of any other individual impurity in the portion of Trospium Chloride taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of each individual impurity from the Sample solution |
| rS | = | = peak response of trospium chloride related compound B from the Standard solution |
| CS | = | = concentration of USP Trospium Chloride Related Compound B RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Trospium Chloride in the Sample solution (mg/mL) |
Acceptance criteria
Individual impurities:
See Impurity Table 1.
Total impurities:
NMT 0.5%
Impurity Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Trospium chloride related compound Ba |
0.7–0.8 | 0.15 |
| Trospium | 1.0 | — |
| Benzilic acid (trospium chloride related compound A) | 1.9–2.8 | 0.15 |
| Any other individual impurity | — | 0.10 |
|
a
(1R,3r,5S)-8-Azabicyclo[3.2.1]octan-3-yl hydroxydiphenylacetate.
|
||
• Procedure 2: Limit of Trospium Chloride Related Compound C
System suitability solution:
0.5 mg/mL of USP Trospium Chloride RS and 0.5 mg/mL of USP Trospium Chloride Related Compound C RS in methanol
Standard solution:
0.1 mg/mL of USP Trospium Chloride Related Compound C RS in methanol
Sample solution:
100 mg/mL of Trospium Chloride in methanol
Chromatographic system
Mode:
TLC
Plate:
0.25-mm layer of chromatographic silica gel mixture
Application volume:
10 µL
Developing distance:
Two-thirds of the length of the plate
Developing solvent system:
Acetonitrile, glacial acetic acid, and hydrochloric acid (45: 1: 3.5)
Spray reagent 1:
Use Dragendorff's TS.
Spray reagent 2:
5 g/L of sodium nitrite in water
System suitability
Sample:
System suitability solution
Suitability requirements
Resolution:
The chromatogram shows two clearly visible and separated spots.
Analysis
Samples:
Standard solution and Sample solution
Allow the spots to dry in a current of warm air until the odor of acetic acid is no longer perceptible. Spray the plate with Spray reagent 1, and subsequently with Spray reagent 2.
Acceptance criteria:
Any spot from the Sample solution corresponding to trospium chloride related compound C is not more intense than the corresponding spot from the Standard solution (0.1%).
SPECIFIC TESTS
• Loss on Drying  731
731 :
Dry the sample at 105
:
Dry the sample at 105 to constant weight: it loses NMT 0.5% of its weight.
to constant weight: it loses NMT 0.5% of its weight.
• pH  791
791
Sample solution:
10 mg/mL of Trospium Chloride in carbon dioxide-free water
Acceptance criteria:
5.0–7.0
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Protect from light. Store at room temperature.
• USP Reference Standards  11
11
USP Trospium Chloride RS
USP Trospium Chloride Related Compound A RS
Benzilic acid.
C14H12O3 228.24

 [76-93-7].
[76-93-7].
Benzilic acid.
C14H12O3 228.24
USP Trospium Chloride Related Compound B RS
Nortropane benzilate;
(1R,3r,5S)-8-Azabicyclo[3.2.1]octan-3-yl hydroxydiphenylacetate.
C21H23NO3 337.41
Nortropane benzilate;
(1R,3r,5S)-8-Azabicyclo[3.2.1]octan-3-yl hydroxydiphenylacetate.
C21H23NO3 337.41
USP Trospium Chloride Related Compound C RS
Azoniaspironortropanol chloride;
(1R,3r,5S)-3-Hydroxyspiro[8-azoniabicyclo[3.2.1]octane-8,1¢-pyrrolidinium] chloride.
C11H20ClNO 217.74
Azoniaspironortropanol chloride;
(1R,3r,5S)-3-Hydroxyspiro[8-azoniabicyclo[3.2.1]octane-8,1¢-pyrrolidinium] chloride.
C11H20ClNO 217.74
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Elena Gonikberg, Ph.D.
Principal Scientific Liaison 1-301-816-8251 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4968
Pharmacopeial Forum: Volume No. 36(4) Page 933