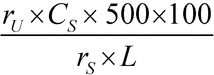Cabergoline Tablets
» Cabergoline Tablets contain not less than 90.0 percent and not more than 110.0 percent of the labeled amount of cabergoline (C26H37N5O2).
Packaging and storage—
Preserve in light-resistant, tight containers, and store at controlled room temperature.
USP Reference standards  11
11 —
—
USP Cabergoline RS
Identification—
The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
Dissolution  711
711 —
—
Medium:
0.1 N hydrochloric acid; 500 mL, degassed with helium.
Apparatus 2:
50 rpm.
Time:
15 minutes.
Determine the amount of C26H37N5O2 dissolved by employing the following procedure.
Mobile phase and Chromatographic system—
Prepare as directed in the Assay.
Standard solution—
Dissolve an accurately weighed quantity of USP Cabergoline RS in Medium to obtain a solution having a known concentration of about 1 µg per mL.
Test solution—
Pass the solution under test through a suitable filter, discarding the first few mL.
Chromatographic system
(see Chromatography  621
621 )—Prepare as directed in the Assay. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the column efficiency is not less than 3000 theoretical plates; and the relative standard deviation for replicate injections is not more than 2%.
)—Prepare as directed in the Assay. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the column efficiency is not less than 3000 theoretical plates; and the relative standard deviation for replicate injections is not more than 2%.
Procedure—
Separately inject equal volumes (about 100 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the peak responses. Calculate the percentage of C26H37N5O2 dissolved by the formula:
in which rU and rS are the responses for the cabergoline peak obtained from the Test solution and the Standard solution, respectively; CS is the concentration of USP Cabergoline RS, in mg per mL, in the Standard solution; 500 is the volume of Medium; and L is the Tablet label claim, in mg per Tablet.
Tolerances—
Not less than 75% (Q) of the labeled amount of C26H37N5O2 is dissolved in 15 minutes.
Uniformity of dosage units  905
905 :
meet the requirements.
:
meet the requirements.
Chromatographic purity—
[note—Prepare solutions immediately before use, and protect from light. ]
Mobile phase—
Prepare as directed in the Assay.
Resolution solution—
To 10 mL of 0.1 M sodium hydroxide add 50 mg of cabergoline. Stir for about 15 minutes. To 1 mL of the suspension add 1 mL of 0.1 M hydrochloric acid, and dilute with Mobile phase to 10 mL. Sonicate until dissolution is complete. The main degradation product obtained is cabergoline related compound A.
Test solution—
Use the Assay preparation, prepared as directed in the Assay.
Chromatographic system
(see Chromatography  621
621 )—Prepare as directed in the Assay. Chromatograph (about 20 µL) of the Resolution solution, and record the peak responses as directed for Procedure. Identify the peaks due to cabergoline related compound A and cabergoline using the relative retention times (RRT) given in Table 1: the resolution, R, between cabergoline and cabergoline related compound A is not less than 3.0.
)—Prepare as directed in the Assay. Chromatograph (about 20 µL) of the Resolution solution, and record the peak responses as directed for Procedure. Identify the peaks due to cabergoline related compound A and cabergoline using the relative retention times (RRT) given in Table 1: the resolution, R, between cabergoline and cabergoline related compound A is not less than 3.0.
Procedure—
Inject a volume (about 100 µL) of the Test solution into the chromatograph, and record the chromatogram. Calculate the percentage of each impurity in the portion of Tablets taken by the formula:
100(ri / rs)
in which ri is the peak area of each impurity obtained from the Test solution; and rs is the sum of the peak areas of all the impurities and the main peak due to cabergoline obtained from the Test solution. Calculate the percentage of total impurities in the portion of Tablets taken by the formula:
100(rt / rs)
in which rt is the sum of the peak areas of all the impurities obtained from the Test solution; and rs is the sum of the peak areas of all the impurities and the main peak due to cabergoline obtained from the Test solution. The relative retention times and limits for cabergoline related compounds A and cabergoline N-oxide are given in Table 1.
Table 1
| Name | RRT | Limit (%) | |
|---|---|---|---|
| Cabergoline related compound A1 | 0.8 | NMT 2.0 | |
| Cabergoline | 1.0 | — | |
| Cabergoline N-oxide2 | 1.4 | NMT 1.0 | |
| Any unspecified degradation product | — | NMT 0.5 | |
| Total | — | NMT 2.5 | |
|
1
(6aR,9R,10aR)-7-(Prop-2-enyl)-4,6,6a,7,8,9,10,10a-octahydroindolo[4,3-fg]quinoline-9-carboxylic acid.
2
(6aR,9R,10aR)-7-Allyl-N-(3-(dimethylazinoyl)propyl)-N-(ethylcarbamoyl)-4,
6,6a,7,8,9,10,10a-octahydroindolo[4,3-fg]quinoline-9-carboxamide. |
|||
Assay—
[note—Prepare solutions immediately before use, and protect from light. ]
Buffer—
Transfer 6.8 g of monobasic potassium phosphate to a 1-L volumetric flask. Dissolve the contents in 900 mL of water. Adjust with phosphoric acid to a pH of 2.0. Dilute with water to volume, add 0.2 mL of triethylamine, and mix well.
Mobile phase—
Prepare a mixture of Buffer and acetonitrile (84:16), and degas. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard preparation—
Dissolve an accurately weighed quantity of USP Cabergoline RS in Mobile phase to obtain a solution having a known concentration of about 0.25 mg per mL. [note—Sonication may be used to aid in the dissolution of cabergoline. ]
Assay preparation—
Grind not fewer than 20 Tablets into a fine powder. Transfer an accurately weighed portion of the powder, equivalent to about 2.5 mg of cabergoline based on the label claim, to a 10-mL volumetric flask. Dilute with Mobile phase to volume, and sonicate until completely dissolved. This solution has a nominal concentration of about 0.25 mg per mL of cabergoline, based on the label claim. [note—The Assay preparation may be passed through a PVDF type filter with a pore size of 0.45 µm prior to analysis. ]
Chromatographic system (see Chromatography  621
621 )—The liquid chromatograph is equipped with a 280-nm detector and a 4.0-mm × 25-cm column that contains 10-µm packing L1. The flow rate is about 1.3 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the column efficiency is not less than 1000 theoretical plates; and the relative standard deviation for replicate injections is not more than 2.0%.
)—The liquid chromatograph is equipped with a 280-nm detector and a 4.0-mm × 25-cm column that contains 10-µm packing L1. The flow rate is about 1.3 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the column efficiency is not less than 1000 theoretical plates; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 100 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the peak responses. Calculate the percentage of the label claim of C26H37N5O2 in the portion of Tablets taken by the formula:
100(CS / CU)(rU / rS)
in which CS is the concentration of USP Cabergoline RS, in mg per mL, in the Standard preparation; CU is the nominal concentration of carbergoline, in mg per mL, in the Assay preparation, based on the label claim; and rU and rS are the peak responses for cabergoline obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ravi Ravichandran, Ph.D.
Principal Scientific Liaison 1-301-816-8330 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
|
| Margareth R.C. Marques, Ph.D.
Senior Scientific Liaison 1-301-816-8106 |
(GCDF2010) General Chapters - Dosage Forms |
USP35–NF30 Page 2426
Pharmacopeial Forum: Volume No. 34(3) Page 572