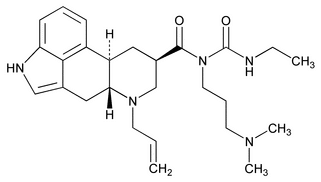
Cabergoline
C26H37N5O2 451.60
Ergoline-8 -carboxamide, N-[3-(dimethylamino)propyl]N-[(ethylamino)carbonyl]-6-(2-propenyl)-;
-carboxamide, N-[3-(dimethylamino)propyl]N-[(ethylamino)carbonyl]-6-(2-propenyl)-;
1-[(6-Allylergolin-8 -yl)carbonyl]-1-[3-(dimethylamino)propyl]-3-ethylurea
-yl)carbonyl]-1-[3-(dimethylamino)propyl]-3-ethylurea


 [81409-90-7].
[81409-90-7].
(ka ber' goe leen).
C26H37N5O2 451.60
Ergoline-8
1-[(6-Allylergolin-8
DEFINITION
Cabergoline contains NLT 98.0% and NMT 102.0% of the labeled amount of C26H37N5O2, calculated on the anhydrous basis.
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
[Note—Prepare solutions immediately before use and protect from light. ]
Buffer:
Dissolve 6.8 g of monobasic potassium phosphate in 900 mL of water, adjust with phosphoric acid to a pH of 2.0, and dilute to 1 L. Add 0.2 mL of triethylamine to the resulting solution and mix.
Mobile phase:
Acetonitrile and Buffer (4:21)
Standard solution:
0.25 mg/mL of USP Cabergoline RS in Mobile phase. [Note—Sonicate if needed. ]
Sample solution:
0.25 mg/mL of Cabergoline in Mobile phase. [Note—Sonicate if needed. ]
Chromatographic system
Mode:
LC
Detector:
UV 280 nm
Column:
4.0-mm × 25-cm; 10 µm packing L1
Flow rate:
1.3 mL/min
Injection size:
100 µL
System suitability
Sample:
Standard solution
Suitability requirements
Column efficiency:
NLT 1000 theoretical plates
Relative standard deviation:
NMT 2.0% for five replicate injections
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C26H37N5O2 in the portion of Cabergoline taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of the Sample solution |
| rS | = | = peak response of the Standard solution |
| CS | = | = concentration of USP Cabergoline RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Cabergoline in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% on the anhydrous basis
IMPURITIES
Organic Impurities
• Procedure
[Note—Prepare solutions immediately before use, and protect from light. ]
Buffer and Mobile phase:
Proceed as directed in the Assay.
System suitability solution:
To 10 mL of 0.1 M sodium hydroxide add 50 mg of Cabergoline and stir for about 15 min. To 1 mL of the suspension add 1 mL of 0.1 M hydrochloric acid, and dilute with Mobile phase to 10.0 mL. Sonicate until dissolution is complete. [Note—The main degradation product obtained is cabergoline related compound A. ]
Sample solution:
0.25 mg/mL of Cabergoline in Mobile phase. [Note—Sonicate if needed. ]
Chromatographic system
Mode:
LC
Detector:
UV 280 nm
Column:
4.0-mm × 25-cm; 10 µm packing L1
Flow rate:
1.3 mL/min
Injection size:
100 µL
System suitability
Sample:
System suitability solution
Suitability requirements
Resolution:
NLT 3.0 between cabergoline and cabergoline related compound A
Analysis
Sample:
Sample solution
Calculate the percentage of each impurity in the portion of Cabergoline taken:
Result = (rU/rT) × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rT | = | = sum of the peak responses for all peaks from the Sample solution |
Acceptance criteria
Individual impurities:
See Impurity Table 1.
Total impurities:
NMT 0.8%
Impurity Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Cabergoline related compound Da | 0.3 | 0.1 |
| Cabergoline related compound Bb | 0.6 | 0.1 |
| Cabergoline related compound Ac | 0.8 | 0.3 |
| Cabergoline | 1.0 | — |
| Cabergoline related compound Cd | 2.9 | 0.3 |
| Any other individual, unidentified impurity | — | 0.10 |
|
a
(6aR,9R,10aR)-N-[3-(Dimethylamino)propyl]-7-(prop-2-enyl)-
4,6,6a,7,8,9,10,10a-octahydroindolo[4,3-fg]quinoline-9-carboxamide.
b
(6aR,9R,10aR)-N9-[3-(Dimethylamino)propyl]-N4-ethyl-7-(prop-2-enyl)
6a,7,8,9,10,10a-hexahydroindolo[4,3-fg]quinoline-4,9(6H)-dicarboxamide.
c
(6aR,9R,10aR)-7-(Prop-2-enyl)-4,6,6a,7,8,9,10,10a-octahydroindolo[4,3-fg]quinoline-9-carboxylic acid.
d
(6aR,9R,10aR)-N9-[3-(Dimethylamino)propyl]-N4-ethyl-N9-(ethylcarbamoyl)-7-(prop2-enyl)-6a,7,8,9,10,10a-hexahydroindolo[4,3-fg]quinoline-4,9(6H)-dicarboxamide.
|
||
SPECIFIC TESTS
• Optical Rotation, Specific Rotation  781S
781S :
:
 77
77 to
to  83
83
Sample solution:
1 mg/mL in alcohol, on the anhydrous basis
• Water Determination, Method I  921
921 :
NMT 0.5%
:
NMT 0.5%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers, protected from light.
• USP Reference Standards  11
11
USP Cabergoline RS
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ravi Ravichandran, Ph.D.
Principal Scientific Liaison 1-301-816-8330 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2425
Pharmacopeial Forum: Volume No. 36(1) Page 75