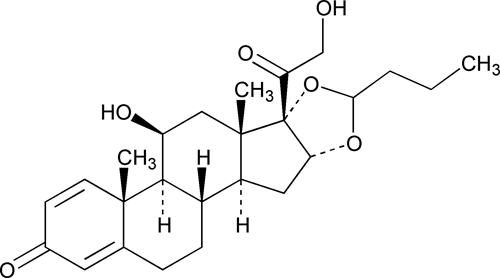
Budesonide
C25H34O6 430.53
Pregna-1,4-diene-3,20-dione, 16 ,17-[1R-butylidenebis(oxy)]-11
,17-[1R-butylidenebis(oxy)]-11 ,21-dihydroxy and pregna-1,4-diene-3,20-dione,16
,21-dihydroxy and pregna-1,4-diene-3,20-dione,16 ,17-[1S-butylidenebis(oxy)]-11
,17-[1S-butylidenebis(oxy)]-11 ,21-dihydroxy;
,21-dihydroxy;
(RS)-11 ,16
,16 ,17,21-Tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with butyraldehyde
,17,21-Tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with butyraldehyde


 [51372-29-3; 51372-28-2; 51333-22-3].
[51372-29-3; 51372-28-2; 51333-22-3].
(bue des' oh nide).
C25H34O6 430.53
Pregna-1,4-diene-3,20-dione, 16
(RS)-11
DEFINITION
Change to read:
Budesonide is a mixture of two epimeric forms, epimer A(C-22S) and epimer B(C-22R). It contains NLT  40.0%
40.0% (RB 1-Jun-2011) and NMT 51.0% of epimer A, and the sum of both epimers is NLT 98.0% and NMT 102.0% of C25H34O6, calculated on the dried basis.
(RB 1-Jun-2011) and NMT 51.0% of epimer A, and the sum of both epimers is NLT 98.0% and NMT 102.0% of C25H34O6, calculated on the dried basis.
[Note—Protect all solutions containing budesonide from light. ]
IDENTIFICATION
• B. Ultraviolet Absorption  197U
197U
Sample solution:
25 µg/mL
Medium:
Methanol
Acceptance criteria:
Meets the requirements
ASSAY
Change to read:
• Procedure
Buffer:
3.17 mg/mL of monobasic sodium phosphate and 0.23 mg/mL of phosphoric acid. The pH is 3.2 ± 0.1.
Mobile phase:
Acetonitrile and Buffer (32:68)
Standard solution:
Dissolve a quantity of USP Budesonide RS in acetonitrile, and dilute quantitatively with Buffer to obtain a solution having a concentration of 0.5 mg/mL, keeping the proportion of acetonitrile in this solution to NMT 30%.
Sample solution:
Dissolve 25 mg of Budesonide in 15 mL of acetonitrile in a 50-mL volumetric flask, and dilute with Buffer to volume.
Chromatographic system
Mode:
LC
Detector:
UV 254 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Flow rate:
1.5 mL/min
Injection size:
20 µL
System suitability
Sample:
Standard solution
[Note—The relative retention time for epimer A is 1.1, with respect to epimer B. ]
Suitability requirements
Resolution:
NLT 1.5 between the two budesonide epimer peaks
Column efficiency:
NLT 5500 theoretical plates, determined from the budesonide epimer B peak
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of epimer A (C25H34O6) in the portion of Budesonide taken:
Result = [rUA/(rUA + rUB)] × 100
| rUA | = | = peak area of epimer A from the Sample solution |
| rUB | = | = peak area of epimer B from the Sample solution |
Calculate the percentage of C25H34O6 in the portion of Budesonide taken:
Result = [(rUA + rUB)/(rSA + rSB)] × (CS/CU) × 100
| rUA | = | = peak area of epimer A from the Sample solution |
| rUB | = | = peak area of epimer B from the Sample solution |
| rSA | = | = peak area of epimer A from the Standard solution |
| rSB | = | = peak area of epimer B from the Standard solution |
| CS | = | = concentration of USP Budesonide RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Budesonide in the Sample solution (mg/mL) |
Acceptance criteria
Epimer A:
 40.0%
40.0% (RB 1-Jun-2011)–51.0% on the dried basis
(RB 1-Jun-2011)–51.0% on the dried basis
Both epimers:
98.0%–102.0% on the dried basis
IMPURITIES
• Procedure 1: Limit of 21-Acetate of Budesonide
Buffer:
3.17 mg/mL of monobasic sodium phosphate and 0.23 mg/mL of phosphoric acid. The pH is 3.2 ± 0.1.
Mobile phase:
Acetonitrile and Buffer (45:55)
Standard solution:
Dissolve a quantity of USP Budesonide RS in acetonitrile, and dilute quantitatively with Buffer to obtain a solution having a concentration of 0.5 mg/mL, keeping the proportion of acetonitrile in this solution to NMT 30%.
Sample solution:
Dissolve 25 mg of Budesonide in 15 mL of acetonitrile in a 50-mL volumetric flask, and dilute with Buffer to volume.
Chromatographic system
Mode:
LC
Detector:
UV 254 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Flow rate:
1.5 mL/min
Injection size:
20 µL
System suitability
Sample:
Standard solution
[Note—The relative retention times for the first eluted epimer of the 21-acetate of budesonide, the second eluted epimer of the 21-acetate of budesonide, the first eluted epimer of budesonide (epimer B), and the second eluted epimer of budesonide (epimer A) are 3.1, 3.2, 1.0, and 1.1, respectively. ]
Suitability requirements
Column efficiency:
NLT 5500 theoretical plates, determined from the budesonide epimer B peak
Analysis
Sample:
Sample solution
Calculate the percentage of the 21-acetate of budesonide in the portion of Budesonide taken:
Result = (rT1/rT2) × 100
| rT1 | = | = sum of the peak areas for the two epimers of the 21-acetate of budesonide |
| rT2 | = | = sum of the peak areas of the two budesonide peaks |
Acceptance criteria:
NMT 0.10% of the 21-acetate of budesonide is found.
• Procedure 2: Limit of 11-Ketobudesonide
Buffer:
3.17 mg/mL of monobasic sodium phosphate and 0.23 mg/mL of phosphoric acid. The pH is 3.2 ± 0.1.
Mobile phase:
Acetonitrile, isopropanol, and Buffer (26:9:65)
Standard solution:
Dissolve a quantity of USP Budesonide RS in acetonitrile, and dilute quantitatively with Buffer to obtain a solution having a concentration of 0.5 mg/mL, keeping the proportion of acetonitrile in this solution to NMT 30%.
Sample solution:
Dissolve 25 mg of Budesonide in 15 mL of acetonitrile in a 50-mL volumetric flask, and dilute with Buffer to volume.
Chromatographic system
Mode:
LC
Detector:
UV 254 nm
Column:
4.6-mm × 15-cm; 3.5-µm packing L1
Column temperature:
50
[Note—Preheat the Mobile phase to 50 . ]
. ]
Flow rate:
1.5 mL/min
Injection size:
20 µL
System suitability
Sample:
Standard solution
[Note—The relative retention times for the two epimers of 11-ketobudesonide are 0.73 and 0.78, respectively; the relative retention times for 21-dehydrobudesonide, 14,15-dehydrobudesonide, and the first eluted epimer of budesonide (epimer B) are 0.68, 0.84, and 1.0, respectively. ]
Suitability requirements
Column efficiency:
NLT 5500 theoretical plates, determined from the budesonide epimer B peak
Analysis
Sample:
Sample solution
Calculate the percentage of 11-ketobudesonide in the portion of Budesonide taken:
Result = (rT1/rT2) × 100
| rT1 | = | = sum of the peak areas for the two ketobudesonide peaks |
| rT2 | = | = sum of the peak areas of the two budesonide peaks |
Acceptance criteria:
NMT 0.2% of 11-ketobudesonide is found.
• Procedure 3
Buffer:
3.17 mg/mL of monobasic sodium phosphate and 0.23 mg/mL of phosphoric acid. The pH is 3.2 ± 0.1.
Mobile phase:
Acetonitrile and Buffer (32:68)
Standard solution:
Dissolve a quantity of USP Budesonide RS in acetonitrile, and dilute quantitatively with Buffer to obtain a solution having a concentration of 0.5 mg/mL, keeping the proportion of acetonitrile in this solution to NMT 30%.
Sample solution:
Dissolve 25 mg of Budesonide in 15 mL of acetonitrile in a 50-mL volumetric flask, and dilute with Buffer to volume.
Chromatographic system
Mode:
LC
Detector:
UV 254 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Flow rate:
1.5 mL/min
Injection size:
20 µL
System suitability
Sample:
Standard solution
Suitability requirements
Column efficiency:
NLT 5500 theoretical plates, determined from the budesonide epimer B peak
Analysis
Sample:
Sample solution
Calculate the percentage of each impurity in the portion of Budesonide taken:
Result = (rU/rT) × 100
| rU | = | = peak area for each impurity |
| rT | = | = sum of the areas of all of the peaks |
Acceptance criteria:
See Table 1.
Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| 16 |
0.11 | 0.2 |
| d-Homobudesonideb | 0.36 | 0.10 |
| 21-Dehydrobudesonide (epimers)c | 0.61; 0.66 | 0.07d |
| 14,15-Dehydrobudesonidee | 0.86 | 0.10 |
| Total specified impurities | — | 0.4f |
| Any other individual impurity | — | 0.10 |
| Total unspecified impurities | — | 0.4 |
|
a
11
b
16
c
16
d
Limit includes both epimers.
e
16
f
Total specified impurities includes 11-ketobudesonide obtained in the test for Limit of 11-Ketobudenoside and the impurities listed above.
|
||
SPECIFIC TESTS
• Microbial Enumeration Tests  61
61 and Tests for Specified Microorganisms
and Tests for Specified Microorganisms  62
62 :
The total aerobic microbial count is NMT 103 cfu/g, and the total combined molds and yeast count is NMT 102 cfu/g.
:
The total aerobic microbial count is NMT 103 cfu/g, and the total combined molds and yeast count is NMT 102 cfu/g.
• Loss on Drying  731
731 :
Dry a sample at 105
:
Dry a sample at 105 to constant weight: it loses NMT 0.3% of its weight.
to constant weight: it loses NMT 0.3% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers. Store at controlled room temperature.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Domenick Vicchio, Ph.D.
Senior Scientific Liaison 1-301-998-6828 |
(SM42010) Monographs - Small Molecules 4 |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2394
Pharmacopeial Forum: Volume No. 36(6) Page 1504