INTRODUCTION
Lipid injectable emulsions for intravenous administration are sterile oil-in-water emulsions of soybean oil, used to provide an ample supply of essential fatty acids, linoleic and linolenic, dispersed with the aid of an emulsifying agent in Water for Injection. Alternatively, soybean oil can be mixed with other suitable oils (neutral triglycerides), such as safflower oil, medium-chain triglycerides (MCT) derived from coconut or palm kernel oils, olive oil, or a marine oil, such as menhaden oil. The size of the lipid droplets is critical: because of mechanical filtration, larger-size fat globules (>5 µm) can be trapped in the lungs. The essential size characteristics of a lipid injectable emulsion for intravenous use include the mean diameter of the lipid droplets and the range of the various droplet diameters distributed around the mean diameter, expressed as the standard deviation. In particular, the amounts of fat globules comprising the large-diameter tail of the globule size distribution are especially important with respect to infusion safety. These two regions of the globule size distribution (mean droplet size and large-diameter tail) must be controlled within specified limits.
The two methods described below are used for determination of the mean lipid droplet diameter and the distribution of large-diameter globule sizes in lipid injectable emulsions. Method I and Method II must be validated. The methods described below to assess the quality of lipid injectable emulsions are to be performed in two stages.
METHOD I—LIGHT-SCATTERING METHOD
For the determination of the mean droplet size of lipid injectable emulsions, either of two common light-scattering techniques may be employed: (1) dynamic light scattering (DLS), also known as photon correlation spectroscopy (PCS), or (2) classical light scattering, based on Mie scattering theory. The DLS, or PCS, technique is based on analyzing the rapid temporal fluctuations in the scattered light intensity that occur due to the random Brownian motion, or diffusion, of any particles, including lipid droplets, suspended in liquid. The intensity is measured at a given angle (usually 90 ) by a suitable detector (e.g., photomultiplier tube) able to measure the rapidly fluctuating scattered light intensity produced by the suspended, diffusing droplets. These scattered intensity data are typically used to calculate the intensity autocorrelation function, which is a simple decaying exponential function in time for droplets of uniform size. A distribution of droplet sizes expresses itself by exponential functions of different decay times. The autocorrelation function generated by the scattered intensity data obtained from a given emulsion can be “inverted” by means of an appropriate deconvolution algorithm in order to obtain the approximate distribution of intensity-weighted diffusion coefficients. From the latter, the distribution of small-diameter droplets is calculated, using the Stokes-Einstein equation and the rules of classical (Mie) light scattering.
) by a suitable detector (e.g., photomultiplier tube) able to measure the rapidly fluctuating scattered light intensity produced by the suspended, diffusing droplets. These scattered intensity data are typically used to calculate the intensity autocorrelation function, which is a simple decaying exponential function in time for droplets of uniform size. A distribution of droplet sizes expresses itself by exponential functions of different decay times. The autocorrelation function generated by the scattered intensity data obtained from a given emulsion can be “inverted” by means of an appropriate deconvolution algorithm in order to obtain the approximate distribution of intensity-weighted diffusion coefficients. From the latter, the distribution of small-diameter droplets is calculated, using the Stokes-Einstein equation and the rules of classical (Mie) light scattering.
By contrast, classical light scattering based on Mie theory analyzes the spatial, rather than temporal, variation of the scattered light intensity by measuring the latter as a function of the scattering angle, typically over a large range of detected angles. The temporal fluctuations in the scattering intensity due to Brownian motion are averaged out in time for each angular measurement. This angular variation occurs as a consequence of the mutual interference of individual scattered waves arriving at the detector with different phases from different points within a given lipid droplet, as well as from different particles. The extent of the angular variation is significant whenever the droplet diameter is not small compared with the wavelength of the laser light (typically 635 nm). Droplets of a given size and refractive index yield a unique curve of scattering intensity vs. angle. A distribution of droplet sizes gives rise to a final angular dependence that represents the superposition, or summation, of individual (different) intensity vs. angle curves. The measured angular dependence of the scattering intensity obtained from a given emulsion sample can be inverted by means of an appropriate deconvolution algorithm and Mie scattering theory in order to obtain the approximate droplet size distribution.
Thus, light scattering, using either dynamic light scattering (i.e., temporal fluctuations due to droplet diffusion) or classical light scattering/Mie theory (i.e., average intensity vs. angle), can provide acceptable results for both the mean diameter and standard deviation of the droplet size distribution. For purposes of illustrating the method used in Method I, a dynamic light-scattering technique is described. For guidance regarding instruments employing classical Mie-theory light scattering, see Light Diffraction Measurement of Particle Size  429
429 .
.
Apparatus—
A suitable DLS/PCS instrument with or without the capability of automatic sample dilution is controlled by validated software and is used to perform the measurement, with the scattering angle typically set at 90 . The intensity-weighted results (mean diameter and standard deviation) are reported, provided it is clearly stated which values are given and that the necessary parameter values required for all requisite calculations are also given.
. The intensity-weighted results (mean diameter and standard deviation) are reported, provided it is clearly stated which values are given and that the necessary parameter values required for all requisite calculations are also given.
Water—
Pass distilled water through a filter of 0.2-µm pore size, and degas by sonication, or use Sterile Water for Injection stored in a glass container.
Standard Preparation—
To a pre-established volume of Water add an appropriate amount of concentrated suspension, containing NIST-traceable polystyrene latex standard particles or other suitable nanospheres. Gently mix the fluids to achieve a homogeneous suspension. The diluted suspension will be slightly turbid in appearance. If the DLS/PCS instrument is equipped with an automatic dilution system, the starting concentrated sample can be analyzed by injection directly into the instrument via a syringe, with further dilution occurring automatically to optimize the droplet concentration for analysis. Alternatively, the sample would require greater manual dilution with Water (typically by at least a factor of 10 over the first dilution), and then this sample would be instilled into a “drop-in” cuvette. The optimum dilution scheme that achieves the proper scattering intensity for the cuvette-based analysis will be determined by the instrument specifications. Thus, the concentration of latex in the final sample must be optimized for the DLS/PCS instrument used. This should be performed separately for three different size standards of approximately 100, 250, and 400 nm (triplicate analyses per size), and the corresponding results of intensity-weighted mean diameter and standard deviation should coincide with the expected values within acceptable errors.
Test Preparation—
To a pre-established volume of water add an appropriate volume of sample from the lipid injectable emulsion. Gently mix the fluids to achieve a homogeneous suspension. The diluted suspension will be slightly turbid in appearance. If the DLS/PCS instrument is equipped with an automatic dilution system, the starting concentrated sample can be analyzed by injection directly into the instrument via a syringe. Further dilution of the sample then occurs automatically to optimize the droplet concentration for analysis, ensuring that it is not so high as to cause artifacts due to multiple scattering or interdroplet interactions. Alternatively, the sample would require greater manual dilution with Water (typically by at least a factor of 10 over the first dilution), and then this sample would be instilled into a “drop-in” cuvette. The optimum dilution scheme that achieves the proper scattering intensity for the cuvette-based analysis will be determined by the instrument specifications. Thus, the concentration of lipid injectable emulsion in the final sample must be optimized for the DLS/PCS instrument used.
System Suitability—
Using the Standard Preparation, measure the intensity-weighted mean particle diameter and the corresponding standard deviation. The system is suitable once the sample temperature has reached equilibration and the results have stabilized and triplicate mean droplet diameter measurements are obtained. The coefficient of variation (CV) should not exceed 10% of the NIST-traceable mean droplet diameter. A larger CV value indicates that the latex microspheres are not suitable as a standard because they either inherently lack uniformity or have become agglomerated to an unacceptable extent. In this case, another standard latex suspension must be selected and tested.
Procedure and Interpretation—
If the DLS/PCS instrument is equipped with an automatic dilution system, use a disposable syringe to load the Standard Preparation or Test Preparation. If no automatic dilution system is used, transfer the appropriately diluted preparation to a cuvette, and place the cuvette in the spectrometer. Allow the sample to equilibrate to a preset controlled temperature close to ambient (between 20 and 25
and 25 , as in the USP definition found in the General Notices under Preservation, Packaging, Storage, and Labeling). Set the instrument scattering angle to 90
, as in the USP definition found in the General Notices under Preservation, Packaging, Storage, and Labeling). Set the instrument scattering angle to 90 , and carry out the measurements. As long as the chi-square (
, and carry out the measurements. As long as the chi-square (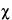 2) goodness-of-fit parameter remains acceptably low (per instrument specifications), the results for the Test Preparation are acceptable. Excessive values of the
2) goodness-of-fit parameter remains acceptably low (per instrument specifications), the results for the Test Preparation are acceptable. Excessive values of the 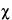 2 parameter suggest that the droplet distribution is not normal and may indicate an unstable emulsion. The intensity-weighted mean droplet diameter (MDD) for lipid injectable emulsions must be less than 500 nm or 0.5 µm, irrespective of the concentration of the dispersed lipid phase.
2 parameter suggest that the droplet distribution is not normal and may indicate an unstable emulsion. The intensity-weighted mean droplet diameter (MDD) for lipid injectable emulsions must be less than 500 nm or 0.5 µm, irrespective of the concentration of the dispersed lipid phase.
METHOD II—MEASUREMENT OF LARGE GLOBULE CONTENT BY LIGHT OBSCURATION OR EXTINCTION METHOD
For determination of the extent of the large-diameter droplet tail (>5 µm) of lipid injectable emulsions, a light obscuration (LO) or light extinction (LE) method that employs a single-particle (globule) optical sizing (SPOS) technique is used. During application of the LE/SPOS technique, passage of a droplet through a thin optical sensing zone results in blockage of a portion of the incident light beam, causing a momentary decrease in the light intensity reaching the “extinction” detector. The magnitude of this decrease in the signal is ideally proportional to the cross-sectional area of the droplet (assumed smaller than the sensing zone thickness), i.e., to the square of the droplet diameter. During optimization of the LE/SPOS instrument for a given emulsion sample, a series of dilutions should be tested to achieve consistency between samples. The goal is to identify a standard range of dilutions that yield consistent data and are most applicable to the formulation tested. Ideally, when comparing different emulsions, the same approximate number of globules are sized each time, and once a standard is achieved, it should be incorporated into the routine sampling plan for validation testing. As long as the fat globule concentration is below the “coincidence limit” of the sensor (determined by the flow cell and optical design), only one globule at most will pass through the sensing zone at any given time, allowing it to be counted and accurately sized (with less than 1% coincidence events). Both the coincidence limit and the optimal flow rate must be known for the LE/SPOS sensor used. Furthermore, it is prudent to perform the large-diameter measurements at a reduced emulsion concentration such that the measurable droplet concentration at threshold of detection (e.g., >1.8 µm) to an upper limit (e.g., 50 µm) is only approximately one-third of the nominal coincidence limit for the sensor used. The resulting single pulse heights are converted to droplet diameters using a standard calibration curve previously constructed from NIST-traceable monosized polystyrene microspheres of known diameters. For additional guidance in the use of the light obscuration methodology, see the general chapter Particulate Matter in Injections  788
788 .
.
Apparatus—
A suitable light obscuration instrument with or without the capability of automatic sample dilution and controlled by a personal computer (PC) is used for the measurement. The number- and volume-weighted particle size distribution data are reported, provided that it is clearly stated which values are given and that the necessary parameter values required for all necessary calculations are also given.
Water—
Pass distilled water through a filter of 0.2-µm pore size, or use Sterile Water for Injection stored in a glass container.
Standard Preparation—
To a pre-established volume of Water add an appropriate amount of concentrated suspension, containing NIST-traceable polystyrene latex standard particles or other suitable microspheres. Gently mix the fluids to achieve a homogeneous suspension. If the light obscuration instrument is equipped with an automatic dilution system, the starting concentrated sample can be analyzed by injection directly into the instrument via a syringe or Teflon sample line. Further dilution of the sample then occurs automatically to optimize the particle concentration for analysis. Alternatively, the sample would require greater manual dilution with water (typically by at least a factor of 10 over the first dilution). The resulting diluted sample is then instilled in an appropriate, clean container, such as a sterile Type I glass container, before being passed through the sensor. In either case the final particle concentration is caused to lie below the coincidence limit of the sensor. The sizing and counting accuracy of the light obscuration instrument should be obtained using two different size standards of approximately 5 and 10 µm (triplicate analyses per size). For the standards after system calibration, set the instrument threshold of detection at 1.8 µm, extended to an upper limit of 50 µm. The corresponding results for the mean diameter should coincide with the expected values, within 10% of the relative standard deviation and 90%–110% size accuracy. In addition, the number of particle counts obtained per mL should also agree within ±10% with the concentration values certified in the documentation provided with each NIST-traceable size standard.
Test Preparation—
To a pre-established volume of water add an appropriate volume of sample from the lipid injectable emulsion (triplicate analyses per sample). Gently mix the fluids to achieve a homogeneous suspension. The diluted emulsion will be slightly turbid in appearance. If the light obscuration instrument is equipped with an automatic dilution system, the starting concentrated sample can be analyzed by injection directly into the instrument via a syringe or nonreactive* Teflon sample line. Further dilution then occurs automatically to optimize the droplet/globule concentration for analysis. Alternatively, the sample would require greater manual dilution with water (typically by at least a factor of 10 over the first dilution). The resulting diluted sample is then instilled in an appropriate, clean container such as a sterile Type I glass container. In either case the final droplet/globule concentration is caused to lie below the coincidence limit of the sensor.
System Suitability—
Perform prior to the test procedure, using the Standard Preparation of a 5- and 10-µm NIST-traceable particle. Measure in triplicate the number-weighted particle diameter and the counts/mL of the standard. The system is suitable when the triplicate mean number-weighted particle diameter measurements are within 10% of the target value, both in terms of repeatability (CV) and closeness to the certified size on the label of the NIST-traceable standard.
Procedure and Interpretation—
If the light obscuration instrument is equipped with an automatic dilution system, use a disposable syringe or Teflon sample line to load the Standard Preparation or Test Preparation. If no automatic dilution system is used, transfer the sample to an appropriate large-volume, clean container such as a sterile Type I glass vessel containing an appropriate volume of water. Allow the sample and water to mix thoroughly to achieve a homogeneous suspension. Set the instrument threshold of detection at 1.8 µm, extended to an upper limit of 50 µm, and vary the concentration and/or data collection times such that there is at least a factor of two in the difference of the total number of globules that measure >5 µm between at least two sample runs. In any case, the number of globules that measure >5 µm should be large enough so that it represents an adequate number of globules that are statistically representative of the large-diameter tail population of the native emulsion. The volume-weighted, large-diameter fat globule limits of the dispersed phase, expressed as the percentage of fat residing in globules larger than 5 µm (PFAT5) for a given lipid injectable emulsion, must not exceed 0.05%.
*
Polyvinyl chloride (PVC) with diethylhexylphthalate (DEHP) has been shown to induce breakdown of lipid injectable emulsions (Drug Product Problem Reporting System. USP File Access No. 11173, May 15, 1991).
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Desmond G. Hunt, Ph.D.
Senior Scientific Liaison 1-301-816-8341 |
(GCDF2010) General Chapters - Dosage Forms |
USP35–NF30 Page 310
Pharmacopeial Forum: Volume No. 36(1) Page 223