Products used for nebulization and intended for pulmonary delivery are characterized using the following tests:
— Drug Substance Delivery Rate and Total Drug Substance Delivered;
— Aerodynamic Assessment of Nebulized Aerosols.
These tests standardize the approach to the assessment of the dose that would be delivered to a patient but are not intended to provide assessment of the nebulizer device itself.1 The mass rather than the number-weighted size distribution is more appropriate to evaluate product performance. Drug substance mass as a function of aerodynamic diameter is more indicative of therapeutic effect within the respiratory tract.
DRUG SUBSTANCE DELIVERY RATE AND TOTAL DRUG SUBSTANCE DELIVERED
These tests are performed to assess the rate of delivery to the patient and the total drug substance delivered to a patient using standardized conditions of volumetric flow rate. Breath-enhanced and breath-actuated nebulizers should be evaluated by a breathing simulator because the output of these types of device is highly dependent on inhalation flow rate. The methodology below describes the use of a standard breathing pattern defined for adults. Should a particular product for nebulization be indicated only for pediatric, i.e., neonate, infant, or child use, then pediatric breathing pattern(s) must be used.2 Breathing patterns are used, rather than continuous flow rates, to provide a more appropriate measure of the mass of drug substance that would be delivered to patients.
Drug substance delivery rate and total drug substance delivered are appropriate characteristics because they allow the mass delivered to be characterized in a standard way regardless of the nebulizer used. Accordingly, the test methodology described below (a) measures the mass of drug substance delivered in the first period (typically 1 min) consequently giving an assessment of drug substance delivery rate and (b) captures the total drug substance mass delivered.
Apparatus
Breathing Simulator—
A commercially available breathing simulator that is able to generate the breathing profiles specified in Table 1 is used for the test. The breathing profile indicated for adults is used unless the medicinal product is specifically intended for use in pediatrics, when alternate patterns should be used, as indicated in Table 1.
Table 1. Breathing Simulator Specification
| Item | Specification | |||
|---|---|---|---|---|
| Adult | Neonate | Infant | Child | |
| Total volume | 500 mL | 25 mL | 50 mL | 155 mL |
| Frequency | 15 cycles/min | 40 cycles/min | 30 cycles/min | 25 cycles/min |
| Waveform | sinusoidal | sinusoidal | sinusoidal | sinusoidal |
| Inhalation:exhalation ratio |
1:1 | 1:3 | 1:3 | 1:2 |
Filter System—
A suitably validated low-resistance filter, capable of quantitatively collecting the aerosol and enabling recovery of the drug substance with an appropriate solvent, is used for the test. The dead volume of the filter casing does not exceed 10% of the tidal volume used in the breath simulation.
Procedure
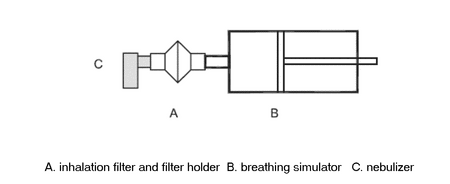
Attach the filter (contained in the filter holder) (A) to the breath simulator (B) according to Figure 1. Fill the nebulizer (C) with the volume of the drug product as specified in the patient instructions. Attach the mouthpiece of the nebulizer to the inhalation filter using a mouthpiece adapter if required, ensuring that connections are airtight. Position the nebulizer in the same orientation as intended for use. This may require tilting the breathing simulator and filter holder. Set the breathing simulator to generate the specified breathing pattern.
Start the breathing simulator and at the beginning of an inhalation cycle, start the nebulizer. Operate the nebulizer for a defined initial time period. The length of the time interval ensures that sufficient drug substance is deposited on the inhalation filter for quantitative analysis. A time of 60 ± 1 s typically enables direct determination of the drug substance delivery rate. The time chosen, usually 60 ± 1 s, must allow sufficient drug substance deposition on the inhalation filter to allow quantitative analysis. If the quantity of drug substance deposited on the inhalation filter in 60 s is insufficient for this analysis, the length of the time interval for aerosol collection can be increased. If the filter is soaked with the product, this time can be decreased. At the end of this initial period, stop the nebulizer.
Place a fresh filter and filter holder in position and continue until nebulization ceases. Interrupt nebulization and exchange filters, if necessary, to avoid filter saturation.
Results
Using a suitable method of analysis, determine the mass of drug substance collected on the filters and filter holders during each time interval. Determine the drug substance delivery rate by dividing the mass of drug substance collected on the first inhalation filter by the time interval used for collection. Determine the total mass of drug substance delivered by summing the mass of drug substance collected on all inhalation filters.
AERODYNAMIC ASSESSMENT OF NEBULIZED AEROSOLS
Nebulized products need to be size-characterized at flow rates lower than the range that is typically used for powder inhalers and metered-dose inhalers. The CEN standard recommends a flow rate of 15 L/min because this value represents a good approximation to the mid-inhalation flow rate achievable by a healthy adult breathing at 500 mL tidal volume.
Although low-angle laser light-scattering instruments (laser diffractometers) can provide rapid size-distribution measurements of nebulizer-generated aerosols, these techniques do not detect the drug substance. Rather, they measure the size distribution of the droplets irrespective of their content. This may not be a problem with homogeneous solutions, but it can result in significant error if the product to be nebulized is a suspension, or if droplet evaporation is significant, as can be the case with certain nebulizer types. Cascade impactors enable the aerosol to be characterized unambiguously in terms of the mass of drug substance as a function of aerodynamic diameter. Laser diffraction may be used if validated against a cascade impaction method.
Apparatus 5 (see general information chapter Aerosols, Nasal Sprays, Metered-Dose Inhalers, and Dry Powder Inhalers  601
601 ), a cascade impactor, has been calibrated at 15 L/min specifically to meet the recommendation of the CEN Standard and is therefore used for this test.3 Determining mass balance in the same way as for powder inhalers and metered-dose inhalers is not straightforward because the dose is being captured as a continuous output and hence is not included. Recovery experiments must be performed as part of method development and validation. Control of evaporation of droplets produced by nebulizers may be critical to avoid bias in the droplet size assessment process. Evaporation can be minimized by cooling the impactor to a temperature of about 5
), a cascade impactor, has been calibrated at 15 L/min specifically to meet the recommendation of the CEN Standard and is therefore used for this test.3 Determining mass balance in the same way as for powder inhalers and metered-dose inhalers is not straightforward because the dose is being captured as a continuous output and hence is not included. Recovery experiments must be performed as part of method development and validation. Control of evaporation of droplets produced by nebulizers may be critical to avoid bias in the droplet size assessment process. Evaporation can be minimized by cooling the impactor to a temperature of about 5 , typically achieved by cooling the impactor in a refrigerator for about 90 min.
, typically achieved by cooling the impactor in a refrigerator for about 90 min.
Typically, at least after each day of use, the apparatus must be fully cleaned, including the inter-stage passageways, because of the greater risk of corrosion caused by the condensation/accumulation of saline-containing droplets on inter-stage metalwork associated with cooling the impactor. All surfaces of the apparatus should be dried after each test, e.g, with compressed air. [Note—The micro-orifice collector (MOC) should not be dried with compressed air. ]
Apparatus
A detailed description of Apparatus 5 and the induction port is contained in  601
601 , and includes details of critical dimensions and the qualification process for the impactor (stage mensuration).
, and includes details of critical dimensions and the qualification process for the impactor (stage mensuration).
A back-up filter in addition to the MOC must be used to ensure quantitative recovery of drug substance from the nebulized aerosol at the specified flow rate of 15 L/min. The filter is located below the MOC (internal filter option), or a filter in holder external to the impactor is used to capture any fine droplets that pass beyond the last size fractionating stage. A pre-separator is not used for testing nebulizer-generated aerosols.
Method Validation
Impactor Stage Overloading—
During method development and validation, confirm that the volume of liquid sampled from the nebulizer does not overload the impactor. Visual inspection of the collection surfaces on stages collecting most of the droplets may reveal streaking if overloading has occurred. This phenomenon is usually also associated with an increase in mass of drug substance collected on the final stage and back-up filter. Reducing the sampling period (To) is the most effective way to avoid overloading in any given system, balancing overloading with analytical sensitivity.
Re-entrainment—
Droplet bounce and re-entrainment are less likely with nebulizer-produced droplets than with solid particles from inhalers, thus coating would not normally be required.
Procedure
Pre-cool the assembled impactor and induction port in a refrigerator (set at about 5 ) for not less than 90 min, and start the determination within about 5 min of impactor removal from the refrigerator. Other methods that maintain the impactor at a constant temperature (e.g, use of a cooling cabinet) can also be employed when validated.
) for not less than 90 min, and start the determination within about 5 min of impactor removal from the refrigerator. Other methods that maintain the impactor at a constant temperature (e.g, use of a cooling cabinet) can also be employed when validated.
Set up the nebulizer with a supply of driving gas (usually air or oxygen), or use a compressor at the pressure and flow rate specified by the manufacturer of the nebulizer. Ensure that the gas supply line does not become detached from the nebulizer when under pressure. Fill the nebulizer with the volume of the medicinal product as specified in the patient instructions.
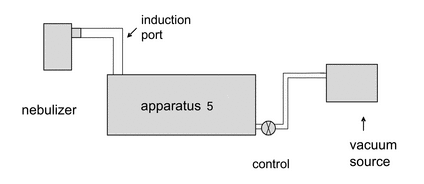
Remove the impactor from the refrigerator. Attach the induction port to the impactor, and connect the outlet of the impactor/external filter to a vacuum source that is capable of withdrawing air through the system at 15 L/min, as specified in Figure 2. Turn on the flow through the impactor.
Connect a flow meter, calibrated for the volumetric flow leaving the meter, to the induction port. Adjust the flow control valve located between the impactor and the vacuum source to achieve a steady flow through the system at 15 L/min (±5%). Remove the flow meter.
Position the nebulizer in the same orientation as intended for use, then attach the mouthpiece of the nebulizer to the induction port, using a mouthpiece adapter if required. Switch on the flow/compressor for the nebulizer. Sample for a predetermined time (To). Once determined, this time (To) must be defined and used in the analytical method for a particular drug product to ensure that mass fraction data can be compared. At the end of the sampling period, switch off the driving gas flow/compressor to the nebulizer, remove the nebulizer from the induction port, and switch off the flow from the vacuum source to the impactor. Dismantle the impactor and, using a suitable method of analysis, determine the mass of drug substance collected in the induction port on each stage and on the back-up filter as described for Apparatus 5 (see  601
601 ). Add the mass of drug substance collected in the MOC to that deposited on the back-up filter/external filter and treat as a single sample for the purpose of subsequent calculations.
). Add the mass of drug substance collected in the MOC to that deposited on the back-up filter/external filter and treat as a single sample for the purpose of subsequent calculations.
Calculate the fine particle mass of the drug substance based on the predetermined time (To). Calculate the mass fraction (Fm,comp) of the drug substance deposited on each component of the impactor, commencing with the induction port and proceeding in order through the impactor, using the following expression:
Fm,comp = mcomp/M
mcomp = mass associated with the component under evaluation;
M = total mass collected by the system.
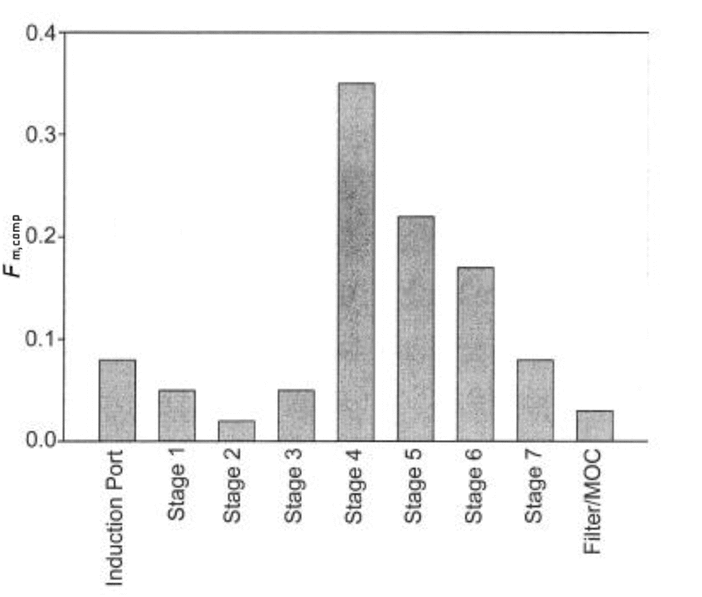
Present Fm,comp in order of location within the measurement equipment, beginning at the induction port and ending with the back-up filter of the impactor (see Figure 3). Fm,comp values for adjacent stages of the impactor may be combined in order to report the mass fraction collected on a group of stages as a single value.
Figure 3. Example of Mass Fraction of Droplets Presented in Terms of Location within the Sampling System.
Determine the cumulative mass-weighted particle-size distribution of the aerosol size-fractionated by the impactor in accordance with the procedure given in  601
601 . Starting at the filter, derive a cumulative mass versus effective cut-off diameter of the respective stages (see Table 2 for the appropriate cut-off diameters at 15 L/min). Plot the cumulative fraction of drug substance versus cut-off diameter in a suitable format, e.g, logarithmic or log-probability format. Where appropriate, use this plot to determine by interpolation the fraction either less than a given size or between an upper and lower size limit.
. Starting at the filter, derive a cumulative mass versus effective cut-off diameter of the respective stages (see Table 2 for the appropriate cut-off diameters at 15 L/min). Plot the cumulative fraction of drug substance versus cut-off diameter in a suitable format, e.g, logarithmic or log-probability format. Where appropriate, use this plot to determine by interpolation the fraction either less than a given size or between an upper and lower size limit.
Table 2. Cut-off Sizes for Apparatus 5 at 15 L/min
| Stage | Cut-off Diameter
(µm) |
|---|---|
| 1 | 14.1 |
| 2 | 8.61 |
| 3 | 5.39 |
| 4 | 3.30 |
| 5 | 2.08 |
| 6 | 1.36 |
| 7 | 0.98 |
If necessary, and as appropriate, use this plot to determine values for the mass median aerodynamic diameter (MMAD) and the geometric standard deviation (GSD).
1
European Standard 13544-1:2001. Respiratory Therapy Equipment. Part 1: Nebulizing systems and their components. European Committee for Standardization. Brussels, Belgium. 2001.
2
Suitable breathing patterns for pediatric use may be found, for example, in Canadian Standard CAN/CSA/Z264.1-02:2002, Spacers and Holding Chambers for Use with Metered Dose Inhalers. Canadian Standards Association, Mississauga, Canada 2002.
3
Marple VA, Olson BA, Santhanakrishnan K, et al. Next generation pharmaceutical impactor: A new impactor for pharmaceutical inhaler testing. Part III: Extension of archival calibration to 15 L/min. J Aerosol Med 2004; 17(4):335–343.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Kahkashan Zaidi, Ph.D.
Senior Scientific Liaison 1-301-816-8269 |
(GCDF2010) General Chapters - Dosage Forms |
USP35–NF30 Page 942
Pharmacopeial Forum: Volume No. 36(2) Page 534