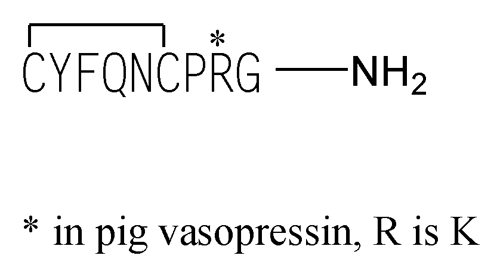Vasopressin
Vasopressin, 8-l-arginine-
C46H65N13O12S2 1056.22
Vasopressin, 8-l-lysine-
» Vasopressin is a polypeptide hormone having the properties of causing the contraction of vascular and other smooth muscles, and of antidiuresis. It is prepared by synthesis or obtained from the posterior lobe of the pituitary of healthy, domestic animals used for food by humans. Its vasopressor activity is not less than 300 USP Vasopressin Units per mg.
Vasopressin is a polypeptide hormone having the properties of causing the contraction of vascular and other smooth muscles, and of antidiuresis. It is prepared by synthesis or obtained from the posterior lobe of the pituitary of healthy, domestic animals used for food by humans. Its vasopressor activity is not less than 300 USP Vasopressin Units per mg.
Packaging and storage—
Preserve in tight containers, preferably of Type I glass, in a refrigerator.
Microbial enumeration tests  61
61 and Tests for specified microorganisms
and Tests for specified microorganisms  62
62 —
The total bacterial count does not exceed 200 cfu per g. For products of animal origin, it meets also the requirements of the tests for absence of Salmonella species and Escherichia coli.
—
The total bacterial count does not exceed 200 cfu per g. For products of animal origin, it meets also the requirements of the tests for absence of Salmonella species and Escherichia coli.
Identification—
A:
The retention time of the vasopressin peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
B:
Mass spectral analysis—
Infusion solution—
Prepare a mixture of acetonitrile, water, and trifluoroacetic acid (80:20:0.08).
Standard solution—
Dissolve an accurately weighed quantity of USP Vasopressin RS in water to obtain a solution having a known concentration of about 1 mg per mL.
Test solution—
Dissolve an accurately weighed quantity of Vasopressin in water to obtain a solution having a known concentration of about 1 mg per mL. [note—The final concentrations of the Standard solution and the Test solution can be adjusted depending on the sensitivity of the mass spectrometer used in the testing.]
Mass spectrometric system
(see Mass Spectrometry  736
736 )—The LC/MS spectrometer is equipped with an infusion system connected to an electrospray interface. The mass spectrometer is operated in the positive ion mode. [note—The infusion system flow rate can be adjusted, as needed. To assist in nebulization, the infusion system can contain a sheathing gas fluid.]
)—The LC/MS spectrometer is equipped with an infusion system connected to an electrospray interface. The mass spectrometer is operated in the positive ion mode. [note—The infusion system flow rate can be adjusted, as needed. To assist in nebulization, the infusion system can contain a sheathing gas fluid.]
Procedure—
Separately inject equal volumes of the Standard solution and the Test solution (about 10 µL) into the infusion system. The flow rate for the infusion system is approximately 0.3 mL per minute. Obtain an optimized mass spectra, following injection. The mass spectra of both the Standard solution and the Test solution should contain peaks with mass-to-charge ratios of 1084 and 543.
Oxytocic activity (for product labeled of animal origin)—
Proceed as directed in the Assay under Oxytocin, except that a suitable dilution of the USP Oxytocin RS will contain approximately 1.2 USP Oxytocin Units per mL of Standard preparation. The oxytocic activity of the Test preparation is not more than 1.2 USP Oxytocin Units per mL.
Ordinary impurities—
The sum of the responses of impurities in the chromatogram of the Assay preparation obtained in the Assay is not more than 5% of the area of the vasopressin peak.
Assay—
Mobile phase—
Dissolve 6.6 g of dibasic ammonium phosphate in about 950 mL of water, and adjust with concentrated phosphoric acid to a pH of 3.0. Dilute with water to 1 L, and mix. To 870 mL of this solution add 130 mL of acetonitrile, and mix. Filter under vacuum through a 0.45-µm nylon membrane. [note—The retention time of the vasopressin peak is very sensitive to small changes in acetonitrile concentration in the Mobile phase.]
Diluent—
Dissolve 5.0 g of chlorobutanol in 5.0 mL of glacial acetic acid, add 5.0 g of alcohol, 1.1 g of sodium acetate, and 1000 mL of water, and mix.
Standard preparation—
Dissolve the entire contents of a vial of USP Vasopressin RS in a known volume of Diluent. [note—The solution may be diluted as necessary to a working concentration range for the assay.]
Assay preparation—
Transfer about 10 mg of Vasopressin, accurately weighed, to a 100-mL volumetric flask. Dissolve in 0.25% glacial acetic acid, and dilute with the same solvent to volume. Mix, and pipet 5.0 mL of this solution into a 100-mL volumetric flask, and dilute with 0.25% glacial acetic acid to volume.
Chromatographic system
(see Chromatography  621
621 )—The liquid chromatograph is equipped with a variable wavelength detector set at 220 mm and a 4.6-mm × 25-cm column that contains packing L1. The flow rate is 1.0 mL per minute. The column is allowed to equilibrate for 1 hour before making the first injection. Determine the suitability of the system (see System Suitability under Chromatography
)—The liquid chromatograph is equipped with a variable wavelength detector set at 220 mm and a 4.6-mm × 25-cm column that contains packing L1. The flow rate is 1.0 mL per minute. The column is allowed to equilibrate for 1 hour before making the first injection. Determine the suitability of the system (see System Suitability under Chromatography  621
621 ) as follows. Inject 20 µL of the Standard preparation into the equilibrated liquid chromatograph, allow about 60 minutes for complete elution, and record the chromatogram as directed for Procedure: the retention time of the vasopressin peak is between 6 and 9 minutes, and is completely resolved from adjacent peaks; the resolution, R, between vasopressin and the nearest adjacent peak is not less than 1.5; and the relative standard deviation for replicate injections is not more than 2.0% for vasopressin.
) as follows. Inject 20 µL of the Standard preparation into the equilibrated liquid chromatograph, allow about 60 minutes for complete elution, and record the chromatogram as directed for Procedure: the retention time of the vasopressin peak is between 6 and 9 minutes, and is completely resolved from adjacent peaks; the resolution, R, between vasopressin and the nearest adjacent peak is not less than 1.5; and the relative standard deviation for replicate injections is not more than 2.0% for vasopressin.
Procedure—
Separately inject equal volumes (about 20 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the potency of Vasopressin, in USP Vasopressin Units per mg, by the formula:
20C(rU / rS)(V / W)
in which V is the volume of sample solution in which the sample was dissolved; and W is the amount, in mg, of vasopressin dissolved in the sample solution; C is the concentration in USP Vasopressin Units per mL in the Standard preparation; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Larry N. Callahan, Ph.D.
Senior Scientist 1-301-816-8385 |
(BBPP05) Biologics and Biotechnology - Proteins and Polysaccharides |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance | |
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance |
USP32–NF27 Page 3849
Pharmacopeial Forum: Volume No. 34(4) Page 994
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.