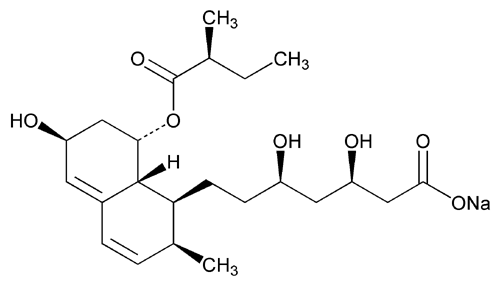
Pravastatin Sodium
1-Naphthaleneheptanoic acid, 1,2,6,7,8,8a-hexahydro-
Sodium (+)-(
» Pravastatin Sodium contains not less than 97.5 percent and not more than 102.0 percent of C23H35NaO7, calculated on the anhydrous and solvent-free basis.
Packaging and storage—
Preserve in tight containers. Store as per labeling instructions. Possible storage conditions could include the following, in the presence of stability data supporting the condition: Store under nitrogen in a cold place. Store at room temperature.
USP Reference standards  11
11 —
—
USP Pravastatin 1,1,3,3-Tetramethylbutylamine RS.
USP Pravastatin Sodium RS .
.
USP Pravastatin Related Compound A RS .
.
USP Pravastatin 1,1,3,3-Tetramethylbutylamine RS.
USP Pravastatin Sodium RS
 .
.
USP Pravastatin Related Compound A RS
 .
.
Identification—
B:
It meets the requirements of the pyroantimonate precipitation test for Sodium  191
191 .
.
Specific rotation  781
781 :
between +150
:
between +150 and +160
and +160 (at 20
(at 20 ), calculated on the anhydrous and solvent-free basis.
), calculated on the anhydrous and solvent-free basis.
Test solution:
5 mg per mL in water.
pH  791
791 :
between 7.2 and 9.0, in a solution (1 in 20).
:
between 7.2 and 9.0, in a solution (1 in 20).
Water, Method I  921
921 :
not more than 4.0%.
:
not more than 4.0%.
Heavy metals, Method II  231
231 :
0.002%.
:
0.002%.
Limit of alcohol
(if present)—
Test solution—
Transfer about 0.2 g of Pravastatin Sodium, accurately weighed, to a 20-mL volumetric flask, dilute with water to volume, and mix. Pipet 5 mL of this solution into a vial fitted with a septum and a crimp cap, add 1 mL of water, seal the vial, and mix. Heat the sealed vial at 80 for 60 minutes.
for 60 minutes.
Standard solution—
Pipet 2 mL of dehydrated alcohol into a 100-mL volumetric flask, dilute with water to volume, and mix. Pipet 10 mL of this solution into a 100-mL volumetric flask, dilute with water to volume, and mix. Pipet 1 mL of this solution into a vial fitted with a septum and a crimp cap, and calculate the amount of alcohol, WA, added, in g, the specific gravity of dehydrated alcohol being 0.79 g per mL. Add 5 mL of the Test solution to the same vial, seal the vial, and mix. Heat the sealed vial at 80 for 60 minutes.
for 60 minutes.
Blank solution—
Pipet 6 mL of water into a vial fitted with a septum and a crimp cap, and seal the vial. Heat the sealed vial at 80 for 60 minutes.
for 60 minutes.
Chromatographic system (see Chromatography  621
621 —
The gas chromatograph is equipped with a flame-ionization detector and a 0.53-mm × 30-m fused silica capillary column coated with a 3-µm film of stationary phase G43. The carrier gas is helium, with a split ratio of 1:5, and flowing with a linear velocity of about 35 cm per second. The chromatograph is programmed as follows. The column temperature is maintained at 40
—
The gas chromatograph is equipped with a flame-ionization detector and a 0.53-mm × 30-m fused silica capillary column coated with a 3-µm film of stationary phase G43. The carrier gas is helium, with a split ratio of 1:5, and flowing with a linear velocity of about 35 cm per second. The chromatograph is programmed as follows. The column temperature is maintained at 40 for 20 minutes, then the temperature is increased at a rate of 10
for 20 minutes, then the temperature is increased at a rate of 10 per minute to 240
per minute to 240 , and maintained at 240
, and maintained at 240 for 20 minutes. The transfer line temperature is maintained at 85
for 20 minutes. The transfer line temperature is maintained at 85 , the injection port temperature is maintained at 140
, the injection port temperature is maintained at 140 , and the detector is maintained at 250
, and the detector is maintained at 250 . Chromatograph the Blank solution, and record the peak responses as directed for Procedure: no interfering peaks are observed.
. Chromatograph the Blank solution, and record the peak responses as directed for Procedure: no interfering peaks are observed.
Procedure—
Separately inject equal volumes (about 1 mL) of headspace gas of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the area responses for the major peaks. Calculate the percentage (w/w) of alcohol in the portion of Pravastatin Sodium taken by the formula:
100(WA / W)(V/5)[rU / (rS – rU)]
in which WA is as defined above; W is the weight, in g, of Pravastatin Sodium taken to prepare the Test solution; V is the volume, in mL, of the Test solution; 5 is the volume, in mL, of the Test solution taken; and rU and rS are the peak area responses of alcohol obtained from the Test solution and the Standard solution, respectively: not more than 3.0% is found.
Chromatographic purity—
[note—The Standard solution and the Test solution are maintained at 15 until injected into the chromatograph.]
until injected into the chromatograph.]
Diluent—
Prepare a mixture of methanol and water (1:1).
Buffer pH 7.0—
Prepare a 0.08 M phosphoric acid solution, adjust with triethylamine to a pH of 7.0, and mix.
Solution A—
Prepare a filtered and degassed mixture of water, Buffer pH 7.0, and acetonitrile (52:30:18).
Solution B—
Prepare a filtered and degassed mixture of acetonitrile, Buffer pH 7.0, and water (60:30:10).
Mobile phase—
Use variable mixtures of Solution A and Solution B as directed for Chromatographic system. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard solution—
Dissolve an accurately weighed quantity of USP Pravastatin 1,1,3,3-Tetramethylbutylamine RS in Diluent, and dilute quantitatively, and stepwise if necessary, with Diluent to obtain a solution having a known concentration of about 1.25 µg of pravastatin 1,1,3,3-tetramethylbutylamine per mL.
System suitability solution—
Dissolve accurately weighed quantities of USP Pravastatin 1,1,3,3-Tetramethylbutylamine RS and USP Pravastatin Related Compound A RS in Diluent to obtain a solution containing about 0.6 mg of USP Pravastatin 1,1,3,3-tetramethylbutylamine RS and 0.001 mg of USP Pravastatin Related Compound A RS per mL. [note—USP Pravastatin Related Compound A RS is a sodium salt of 3 -hydroxyisocompactin acid.]
-hydroxyisocompactin acid.]
Test solution—
Transfer about 50 mg of Pravastatin Sodium, accurately weighed, to a 100-mL volumetric flask, dissolve in and dilute with Diluent to volume, and mix.
Chromatographic system (see Chromatography  621
621 —
The liquid chromatograph is equipped with a 238-nm detector and a 4.6-mm × 7.5-cm column that contains 3.5-µm packing L1. Alternatively, a 4.0-mm × 10-cm column that contains 3-µm packing L1 can be used. The flow rate is about 1 mL per minute. The chromatograph is programmed as follows.
—
The liquid chromatograph is equipped with a 238-nm detector and a 4.6-mm × 7.5-cm column that contains 3.5-µm packing L1. Alternatively, a 4.0-mm × 10-cm column that contains 3-µm packing L1 can be used. The flow rate is about 1 mL per minute. The chromatograph is programmed as follows.
Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the relative retention times are about 1.0 for pravastatin and 1.1 for pravastatin related compound A; and the resolution, R, between pravastatin and pravastatin related compound A is not less than 2.0. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 10.0%.
| Time (minutes) |
Solution A
( %) |
Solution B
(%) |
Elution |
| 0–3.0 | 100 | 0 | isocratic |
| 3.0–26.5 | 100®0 | 0®100 | linear gradient |
| 26.5–26.6 | 0®100 | 100®0 | linear gradient |
| 26.6–30.0 | 100 | 0 | re-equilibration |
Procedure—
Separately inject equal volumes (about 10 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, identify the impurities listed in Table 1, and measure the peak responses. Calculate the percentage of each impurity in the portion of Pravastatin Sodium taken by the formula:
100×(446.51/553.78)C(V/W)(ri / rS)
in which 446.51 and 553.78 are the molecular weights of pravastatin sodium and pravastatin 1,1,3,3-tetramethylbutylamine, respectively; C is the concentration, in mg per mL, of pravastatin 1,1,3,3-tetramethylbutylamine in the Standard solution; V is the volume, in mL, of the Test solution; W is the weight, in mg, of Pravastatin Sodium taken to prepare the Test solution; ri is the peak response for each impurity obtained from the Test solution; and rS is the pravastatin peak response obtained from the Standard solution. In addition to not exceeding the limits for each impurity specified in Table 1, not more than 0.1% of any other individual impurity is found, and not more than 0.6% of total impurities is found.
Table 1
| Name | Relative Retention Time |
Limit (%) |
| 3¢¢-Hydroxypravastatin | 0.33 | 0.2 |
| 6¢-Epipravastatin | 0.92 | 0.3 |
| 3 |
1.1 | 0.2 |
| Pentanoyl impurity2 | 1.2 | 0.2 |
| Pravastatin lactone | 1.8 | 0.2 |
| Compactin | 3.1 | 0.2 |
|
1
Sodium (3R,5R)-3,5-dihydroxy-7-[(1S,2S,3S,8S,8aR)-3-hydroxy-2-methyl-8-[[(2S)-2-methylbutanoyl]oxy]-1,2,3,7,8,8a-hexahydronaphthalen-1-yl]heptanoate (pravastatin related compound A).
|
||
|
2
(3R,5R)-3,5-dihydroxy-7-[(1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-[[(2S)-2-methylpentanoyl]oxy]-1,2,6,7,8,8a-hexahydronaphthalen-1-yl]heptanoic acid.
|
||
Assay—
Solution A—
Prepare a 0.08 M phosphoric acid solution, adjust with a 25% sodium hydroxide solution to a pH of 5.0, mix, filter, and degas.
Solution B—
Use acetonitrile.
Mobile phase—
Use variable mixtures of Solution A and Solution B as directed for Chromatographic system. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard preparation—
Dissolve an accurately weighed quantity of USP Pravastatin 1,1,3,3-Tetramethylbutylamine RS in methanol, and dilute quantitatively, and stepwise if necessary, with methanol to obtain a solution having a known concentration of about 0.25 mg of pravastatin 1,1,3,3-tetramethylbutylamine per mL.
System suitability preparation—
Dissolve accurately weighed quantities of USP Pravastatin 1,1,3,3-Tetramethylbutylamine RS and USP Pravastatin Related Compound A RS in methanol to obtain a solution containing about 0.25 mg of USP Pravastatin 1,1,3,3-Tetramethylbutylamine RS and 0.001 mg of USP Pravastatin Related Compound A RS per mL.
Assay preparation—
Transfer about 20 mg of Pravastatin Sodium, accurately weighed, to a 100-mL volumetric flask, dissolve in and dilute with methanol to volume, and mix.
Chromatographic system (see Chromatography  621
621 —
The liquid chromatograph is equipped with a 238-nm detector and a 4.0-mm × 10-cm column that contains 3-µm packing L1. The flow rate is about 1 mL per minute. The chromatograph is programmed as follows.
—
The liquid chromatograph is equipped with a 238-nm detector and a 4.0-mm × 10-cm column that contains 3-µm packing L1. The flow rate is about 1 mL per minute. The chromatograph is programmed as follows.
Chromatograph the System suitability preparation, and record the peak responses as directed for Procedure: the relative retention times are about 1.0 for pravastatin and 1.2 for pravastatin related compound A; the resolution, R, between pravastatin and pravastatin related compound A is not less than 1.2; and the relative standard deviation for replicate injections for the pravastatin peak is not more than 2.0%.
| Time (minutes) |
Solution A
(%) |
Solution B
(%) |
Elution |
| 0–7.0 | 80®72 | 20®28 | linear gradient |
| 7.0–10.0 | 72®50 | 28®50 | linear gradient |
| 10.0–17.0 | 50 | 50 | isocratic |
| 17.0–17.1 | 50®80 | 50®20 | linear gradient |
| 17.1–20.0 | 80 | 20 | re-equilibration |
Procedure—
Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the pravastatin peaks. Calculate the quantity, in mg, of C23H35NaO7 in the portion of Pravastatin Sodium taken by the formula:
(446.51/553.78)VC(rU / rS)
in which 446.51 and 553.78 are the molecular weights of pravastatin sodium and pravastatin 1,1,3,3-tetramethylbutylamine, respectively; V is the volume, in mL, of the Assay preparation; C is the concentration, in mg per mL, of pravastatin 1,1,3,3-tetramethylbutylamine in the Standard preparation; and rU and rS are the responses of the pravastatin peak obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Elena Gonikberg, Ph.D.
Senior Scientist 1-301-816-8251 |
(MDGRE05) Monograph Development-Gastrointestinal Renal and Endocrine |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 3359
Pharmacopeial Forum: Volume No. 34(2) Page 294
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.