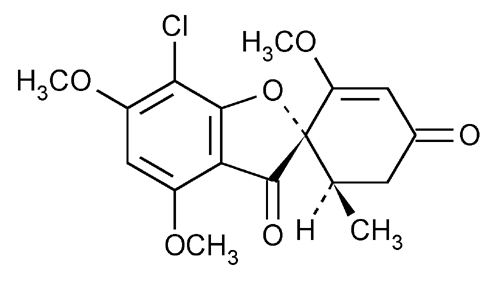
Griseofulvin
Spiro[benzofuran-[2](3H),1¢-2cyclohexene]-3,4¢-dione, 7-chloro-2¢,4,6-trimethoxy-6¢-methyl-, (1¢S-trans)-.
7-Chloro-2¢,4,6-trimethoxy-6¢
» Griseofulvin has a potency of not less than 900 µg of C17H17ClO6 per mg.
Packaging and storage—
Preserve in tight containers.
Identification—
B:
The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
Crystallinity  695
695 :
meets the requirements.
:
meets the requirements.
Loss on drying  731
731 —
Dry about 100 mg, accurately weighed, in a capillary-stoppered bottle in vacuum at a pressure not exceeding 5 mm of mercury at 60
—
Dry about 100 mg, accurately weighed, in a capillary-stoppered bottle in vacuum at a pressure not exceeding 5 mm of mercury at 60 for 3 hours: it loses not more than 1.0% of its weight.
for 3 hours: it loses not more than 1.0% of its weight.
Residue on ignition  281
281 :
not more than 0.2%.
:
not more than 0.2%.
Permeability diameter—
Determine the apparent particle size in µm by the air-permeation method, using a suitable subsieve sizer. Weigh 1.819 ± 0.001 g of Griseofulvin, and transfer to the compression tube of the apparatus. Compact with moderate pressure so that a uniform porosity is achieved. Pass dry compressed air through the tube, and measure the air pressure with a water manometer. Read the porosity, and calculate the apparent particle size from the instrument equation. Repeat the porosity readings at successively higher degrees of compaction until the apparent particle size reaches a minimum value. Calculate the observed permeability diameter, in square meters per g, taken by the formula:
Concomitantly determine the observed permeability diameter of a similar preparation of USP Griseofulvin Permeability Diameter RS. Calculate the permeability diameter of the Griseofulvin taken by the formula:
6 / (1.455MF)
in which M is the minimum apparent particle size; and F is a factor, obtained from the accompanying table, interpolation being used if necessary, to correct the apparent particle size to the true particle size at a given porosity reading.
| Porosity
Reading |
F | Porosity
Reading |
F |
| 0.80 | 1.3771 | 0.56 | 1.7353 |
| 0.76 | 1.4142 | 0.52 | 1.8528 |
| 0.72 | 1.4573 | 0.48 | 2.0076 |
| 0.68 | 1.5082 | 0.44 | 2.2203 |
| 0.64 | 1.5690 | 0.40 | 2.5298 |
| 0.60 | 1.6432 |
OU (AS / OS)
in which OU is the observed permeability diameter of the specimen, AS is the assigned permeability diameter of USP Griseofulvin Permeability Diameter RS, and OS is the observed permeability diameter of USP Griseofulvin Permeability Diameter RS: it is between 1.3 and 1.7 square meters per g.
Heavy metals, Method II  231
231 :
0.0025%.
:
0.0025%.
Assay—
Mobile phase—
Prepare a suitable filtered mixture of water, acetonitrile, and tetrahydrofuran (60:35:5). Degas for 5 minutes before use, and stir continuously during use. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard preparation—
Dissolve an accurately weighed quantity of USP Griseofulvin RS in methanol to obtain a solution having a known concentration of about 1.25 mg per mL. Transfer 5.0 mL of this solution to a 50-mL volumetric flask, dilute with Mobile phase to volume, and mix. This solution contains about 0.125 mg of USP Griseofulvin RS in each mL.
Assay preparation—
Transfer about 62 mg of Griseofulvin, accurately weighed, to a 50-mL volumetric flask, dissolve in and dilute with methanol to volume, and mix. Transfer 5.0 mL of this solution to a 50-mL volumetric flask, dilute with Mobile phase to volume, and mix.
Chromatographic system
(see Chromatography  621
621 )—The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 25-cm column that contains packing L10. The flow rate is about 1 mL per minute. The relative standard deviation for replicate injections of Standard preparation is not more than 2.0%.
)—The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 25-cm column that contains packing L10. The flow rate is about 1 mL per minute. The relative standard deviation for replicate injections of Standard preparation is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 20 µL) of the Standard preparation and the Assay preparation into the chromatograph, and measure the peak responses for the major peaks. Calculate the quantity, in µg of C17H17ClO6, in each mg of the Griseofulvin taken by the formula:
500(CP / WU)(rU / rS)
in which C is the concentration, in mg per mL, of USP Griseofulvin RS in the Standard preparation; P is the content, in µg of C17H17ClO6 per mg, of USP Griseofulvin RS; WU is the quantity, in mg, of Griseofulvin taken; and rU and rS are the griseofulvin peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Ahalya Wise, M.S.
Scientist 1-301-816-8161 |
(MDANT05) Monograph Development-Antibiotics |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2531
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.