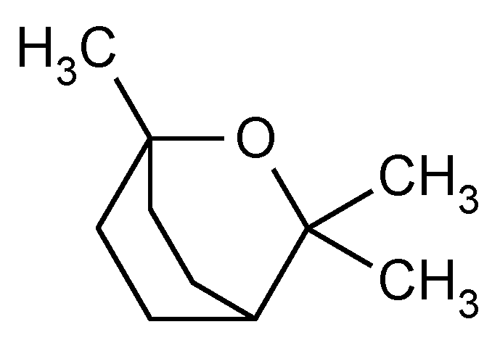
Eucalyptol
» Eucalyptol is obtained from oil of eucalyptus and from other sources. It contains not less than 98.0 percent and not more than 100.0 percent of C10H18O.
Packaging and storage—
Preserve in tight containers.
Identification—
Add 1 mL of phosphoric acid to 1 mL of Eucalyptol contained in a test tube maintained in an ice bath. A solid white crystalline mass is formed, from which eucalyptol separates upon addition of warm water.
Specific gravity  841
841 :
between 0.921 and 0.924.
:
between 0.921 and 0.924.
Congealing temperature  651
651 :
not lower than 0
:
not lower than 0 .
.
Refractive index  831
831 :
between 1.455 and 1.460 at 20
:
between 1.455 and 1.460 at 20 .
.
Limit of phenols—
A:
Shake 5 mL with 5 mL of sodium hydroxide TS: the volume of Eucalyptol is not diminished.
B:
Shake 1 mL with 20 mL of water, and allow the liquids to separate. To 10 mL of the water layer, add 1 drop of ferric chloride TS: the mixture develops no violet color.
Assay—
Assay preparation—
Transfer about 90 mg of eucalyptol, accurately weighed, to a 100-mL volumetric flask, dilute with methanol to volume, and mix.
System suitability solution—
Prepare a solution of limonene and eucalyptol in methanol containing 0.2 mg per mL and 0.9 mg per mL, respectively.
Chromatographic system
(see Chromatography  621
621 )—The gas chromatograph is equipped with a flame-ionization detector and a 0.32-mm × 60-m fused-silica capillary column coated with phase G16. The temperatures of the injection port and the detector block are maintained at 250
)—The gas chromatograph is equipped with a flame-ionization detector and a 0.32-mm × 60-m fused-silica capillary column coated with phase G16. The temperatures of the injection port and the detector block are maintained at 250 . The carrier gas is helium adjusted to a column head pressure of 30 psi. The split flow rate is about 50 mL per minute. The column temperature is initially maintained at 60
. The carrier gas is helium adjusted to a column head pressure of 30 psi. The split flow rate is about 50 mL per minute. The column temperature is initially maintained at 60 , then increased to 200
, then increased to 200 at the rate of 6
at the rate of 6 per minute, beginning at the time of injection. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the resolution, R, between limonene and eucalyptol is not less than 2.0; and the column efficiency determined from the eucalyptol peak is not less than 150,000 theoretical plates.
per minute, beginning at the time of injection. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the resolution, R, between limonene and eucalyptol is not less than 2.0; and the column efficiency determined from the eucalyptol peak is not less than 150,000 theoretical plates.
Procedure—
Separately inject equal volumes (about 1 µL) of the Assay preparation and of methanol into the chromatograph, record the chromatograms, and measure the areas of the peaks. Identify by their retention times any peaks present in the chromatogram obtained from the Assay preparation that correspond to those in the chromatogram of methanol. Calculate the percentage of C10H18O in the portion of Eucalyptol taken by the formula:
100(rU / rT)
in which rU is the eucalyptol peak response obtained from the Assay preparation; and rT is the sum of the peak responses obtained from the Assay preparation, other than the responses corresponding to those in the chromatogram of methanol.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Clydewyn M. Anthony, Ph.D.
Scientist 1-301-816-8139 |
(MDCCA05) Monograph Development-Cough Cold and Analgesics |
USP32–NF27 Page 2340