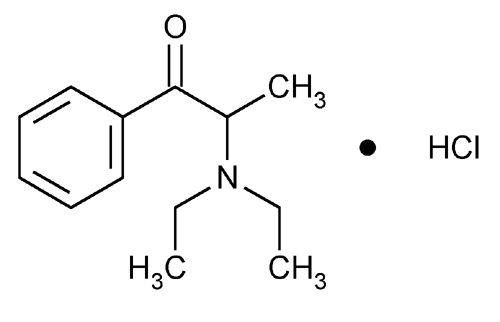
Diethylpropion Hydrochloride
1-Propanone, 2-(diethylamino)-1-phenyl-, hydrochloride.
2-(Diethylamino)propiophenone hydrochloride
» Diethylpropion Hydrochloride contains not less than 97.0 percent and not more than 103.0 percent of C13H19NO·HCl, calculated on the anhydrous basis. It may contain tartaric acid as a stabilizer.
Packaging and storage—
Preserve in well-closed, light-resistant containers.
Labeling—
The label indicates whether it contains tartaric acid as a stabilizer.
Identification—
B:
The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that of the Standard preparation as obtained in the Assay.
C:
A solution (1 in 100) responds to the tests for Chloride  191
191 .
.
Water, Method I  921
921 :
not more than 0.5%.
:
not more than 0.5%.
Secondary amines—
Dissolve 100 mg in 2 mL of methylene chloride in a centrifuge tube. Transfer to a second tube 2 mL of a Standard solution of diethylamine hydrochloride (dried at 105 for 2 hours before being used) in methylene chloride having a known concentration of 250 µg per mL. Treat each solution as follows. Extract with 2 mL of a buffer solution containing 5.7 g of sodium carbonate and 3.0 g of sodium bicarbonate per 100 mL of water. Centrifuge, if necessary, to clarify the upper phase, and immediately transfer 0.5 mL of it to a spot plate. Immediately add 2 drops of acetaldehyde TS, and then, in rapid succession, add 1 drop of sodium nitroferricyanide solution (1 in 100) to each spot. Immediately and simultaneously stir both spots to mix the reagents: any blue color produced within 3 minutes by the test solution is not more intense than that of the Standard solution (not more than 0.5% of secondary amines as diethylamine hydrochloride).
for 2 hours before being used) in methylene chloride having a known concentration of 250 µg per mL. Treat each solution as follows. Extract with 2 mL of a buffer solution containing 5.7 g of sodium carbonate and 3.0 g of sodium bicarbonate per 100 mL of water. Centrifuge, if necessary, to clarify the upper phase, and immediately transfer 0.5 mL of it to a spot plate. Immediately add 2 drops of acetaldehyde TS, and then, in rapid succession, add 1 drop of sodium nitroferricyanide solution (1 in 100) to each spot. Immediately and simultaneously stir both spots to mix the reagents: any blue color produced within 3 minutes by the test solution is not more intense than that of the Standard solution (not more than 0.5% of secondary amines as diethylamine hydrochloride).
Free bromine—
One drop of a solution (1 in 10) produces no discoloration when placed upon starch iodide paper.
Limit of hydrobromic acid and bromide—
To 10 mL of a solution (1 in 10) add 1 mL of sodium hydroxide solution (1 in 10), extract with about 25 mL of chloroform, and discard the chloroform extract. Add 1 mL of 6 N hydrochloric acid, 0.5 mL of chloroform, and 0.5 mL of freshly prepared chloramine T solution (1 in 10), and shake vigorously: no yellow or brown-red color is produced in the chloroform layer.
Chromatographic purity—
Phosphate buffer—
Dissolve 136.1 g of monobasic potassium phosphate in 900 mL of water, add 3.2 mL of phosphoric acid, dilute with water to 1000 mL, and mix.
Diluent—
Prepare a mixture of water, Phosphate buffer, and acetonitrile (8:1:1).
Mobile phase—
Mix 100 mL of acetonitrile, 100 mL of Phosphate buffer, 7.0 mL of diethylamine, and sufficient water to make 1 L. Filter, and degas before use. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Test preparation—
Transfer 100 mg of Diethylpropion Hydrochloride, accurately weighed, to a 50-mL volumetric flask, dissolve in about 40 mL of Diluent, add Diluent to volume, and mix.
Standard preparation—
Dissolve an accurately weighed quantity of USP Diethylpropion Hydrochloride RS in Diluent, and dilute quantitatively, and stepwise if necessary, with Diluent to obtain a solution having a known concentration of about 0.01 mg per mL.
System suitability solution—
Prepare a solution in Diluent containing about 25 µg of 2-ethylaminopropiophenone hydrochloride and 50 µg of USP Diethylpropion Hydrochloride RS per mL.
Chromatographic system
(see Chromatography  621
621 )—The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 15-cm column that contains packing L11. The flow rate is about 1 mL per minute. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.5 for 2-ethylaminopropiophenone and 1.0 for diethylpropion, and the resolution, R, between the 2-ethylaminopropiophenone and diethylpropion peaks is not less than 6.0. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 1.0%.
)—The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 15-cm column that contains packing L11. The flow rate is about 1 mL per minute. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.5 for 2-ethylaminopropiophenone and 1.0 for diethylpropion, and the resolution, R, between the 2-ethylaminopropiophenone and diethylpropion peaks is not less than 6.0. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 1.0%.
Procedure—
Separately inject equal volumes (about 20 µL) of the Standard preparation and the Test preparation into the chromatograph, record the chromatograms, and measure the peak responses. The sum of all of the peak responses, excluding the solvent peak responses and the diethylpropion response, from the Test preparation is not greater than the diethylpropion response from the Standard preparation (0.5%).
Assay—
Phosphate buffer—
Dissolve 136.1 g of monobasic potassium phosphate in 900 mL of water, add 4.3 mL of phosphoric acid, dilute with water to 1000 mL, and mix.
Mobile phase—
Prepare a suitable mixture of water, acetonitrile, Phosphate buffer, and 1.0 M sodium nitrate (730:200: 50:20), filter through a membrane filter (0.7-µm or finer porosity), and degas. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard preparation—
Dissolve an accurately weighed quantity of USP Diethylpropion Hydrochloride RS in Mobile phase, and dilute quantitatively, and stepwise if necessary, with Mobile phase to obtain a solution having a known concentration of about 40 µg per mL.
Assay preparation—
Transfer about 100 mg of Diethylpropion Hydrochloride, accurately weighed, to a 250-mL volumetric flask, dissolve in Mobile phase, dilute with Mobile phase to volume, and mix. Transfer 10.0 mL of this solution to a 100-mL volumetric flask, dilute with Mobile phase to volume, and mix.
System suitability preparation—
Prepare a solution in Mobile phase containing about 200 µg of benzoic acid and 40 µg of USP Diethylpropion Hydrochloride RS per mL.
Chromatographic system
(see Chromatography  621
621 )—The liquid chromatograph is equipped with a 254-nm detector and a 4-mm × 30-cm column that contains packing L1. The flow rate is about 1.5 mL per minute. Chromatograph the System suitability preparation, and record the peak responses as directed for Procedure: the relative retention times are about 0.5 for diethylpropion hydrochloride and 1.0 for benzoic acid, and the resolution, R, between the diethylpropion hydrochloride and benzoic acid peaks is not less than 2.0. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 1.0%.
)—The liquid chromatograph is equipped with a 254-nm detector and a 4-mm × 30-cm column that contains packing L1. The flow rate is about 1.5 mL per minute. Chromatograph the System suitability preparation, and record the peak responses as directed for Procedure: the relative retention times are about 0.5 for diethylpropion hydrochloride and 1.0 for benzoic acid, and the resolution, R, between the diethylpropion hydrochloride and benzoic acid peaks is not less than 2.0. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 1.0%.
Procedure—
Separately inject equal volumes (about 50 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of C13H19NO·HCl in the portion of Diethylpropion Hydrochloride taken by the formula:
2.5C(rU / rS)
in which C is the concentration, in µg per mL, of USP Diethylpropion Hydrochloride RS in the Standard preparation, and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Clydewyn M. Anthony, Ph.D.
Scientist 1-301-816-8139 |
(MDCCA05) Monograph Development-Cough Cold and Analgesics |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2136
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.