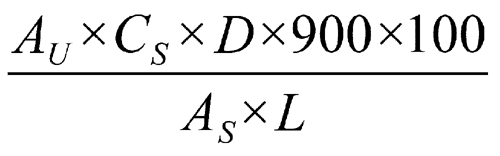Add the following:
» Cefdinir Capsules contain not less than 90.0 percent and not more than 110.0 percent of the labeled amount of C14H13N5O5S2.
Packaging and storage—
Preserve in tight, light-resistant containers, and store at controlled room temperature.
USP Reference standards  11
11 —
—
USP Cefdinir RS.
USP Cefdinir Related Compound A RS.
USP Cefdinir Related Compound B RS.
USP Cefdinir RS.
USP Cefdinir Related Compound A RS.
USP Cefdinir Related Compound B RS.
Identification—
0.1 M Phosphate buffer solution—
Prepare as directed in the Assay.
Blank solution:
0.1 M Phosphate buffer solution.
Standard solution—
Prepare a solution containing about 0.01 mg per mL of USP Cefdinir RS in 0.1 M Phosphate buffer solution.
Sample solution—
Prepare a solution containing an amount equivalent to about 0.01 mg per mL of Cefdinir in 0.1 M Phosphate buffer solution, and pass through a suitable filter.
Procedure—
Compare the spectrum obtained from the Sample solution using a 1-cm cell to that obtained from the Standard solution, using the Blank solution to zero the instrument: the UV absorption spectrum of the Sample solution exhibits maxima and minima at the same wavelengths as that of a Standard solution concomitantly measured.
B:
The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
Dissolution  711
711 —
—
Medium:
0.05 M phosphate buffer, pH 6.8; 900 mL.
Apparatus 2:
50 rpm.
Time:
30 minutes.
Standard solution—
Dissolve an accurately weighed quantity of USP Cefdinir RS in Medium to obtain a solution having a concentration of about 0.33 mg per mL.
Test solution—
Pass a portion of the solution under test through a suitable 0.45-µm filter. If necessary, dilute a portion of each filtered sample with Medium to obtain a solution that has a theoretical concentration of about 0.33 mg per mL of cefdinir, considering complete release of the label claim.
Blank solution—
Dissolve one empty gelatin capsule shell in 100 mL of Medium, dilute with Medium to 900 mL, and filter if necessary.
Procedure—
Determine the amount of C14H13N5O5S2 dissolved by UV absorption at the wavelength of maximum absorbance at 290 nm on portions of the Test solution, in comparison with the Standard solution, using a 1-cm cell and Blank solution as blank. Calculate the percentage of C14H13N5O5S2 dissolved by the formula:
in which AU and AS are the absorbances obtained from the Test solution and the Standard solution, respectively; CS is the concentration, in mg per mL, of the Standard solution; D is the dilution factor of the Test solution; 900 is the volume, in mL, of Medium; 100 is the conversion factor to percentage; and L is the capsule label claim, in mg.
Tolerances—
Not less than 80% (Q) of the labeled amount of C14H13N5O5S2 is dissolved in 30 minutes.
Uniformity of dosage units  905
905 :
meet the requirements.
:
meet the requirements.
Related compounds—
0.1 M Phosphate buffer solution—
solution A—
Dissolve 14.2 g of sodium phosphate, dibasic, anhydrous, in water, and dilute with water to 1000.0 mL.
solution B—
Dissolve 6.8 g of potassium phosphate, monobasic, in water, and dilute with water to 500.0 mL.
solution C—
Combine 1000 mL of Solution A with 500 mL of Solution B. Verify the pH of 7.0 ± 0.1, and adjust, if necessary using Solution A or Solution B.
Dilute phosphoric acid solution—
Dilute phosphoric acid (1 in 10) with water, and mix.
0.1 M Disodium ethylenediaminetetraacetate (EDTA)—
Transfer about 3.72 g of disodium ethylenediaminetetraacetate into a 100-mL volumetric flask. Dissolve in and dilute with water to volume, and mix.
0.1% Tetramethylammonium hydroxide solution—
Dilute 20 mL of tetramethylammonium hydroxide (10% in water) with water to make 2000 mL, and mix. Adjust with Dilute phosphoric acid solution to a pH of 5.5 ± 0.1.
Mobile phase A—
Transfer 0.4 mL of 0.1 M EDTA to 1000 mL of 0.1% Tetramethylammonium hydroxide solution, and mix.
Mobile phase B—
Mix 250 mL of 0.1% Tetramethylammonium hydroxide solution, 150 mL of acetonitrile, and 100 mL of methanol. Add 0.2 mL of 0.1 M EDTA, and mix.
Mobile phase—
Use variable mixtures of Mobile phase A and Mobile phase B as directed in the Chromatographic system.
Standard solution 1—
Dissolve an appropriate amount of USP Cefdinir RS in 0.1 M Phosphate buffer solution (Solution C) to obtain a solution having a known concentration of about 0.75 mg per mL of USP Cefdinir RS. Dilute with 0.1% Tetramethyl ammonium hydroxide solution an appropriate amount of this solution, stepwise, if necessary, to obtain a solution having a known concentration of about 15 µg per mL.
Standard solution 2—
Dissolve an appropriate quantity of USP Cefdinir Related Compound A RS in 0.1% Tetramethylammonium hydroxide solution to obtain a solution having a known concentration of about 0.04 mg per mL.
Standard solution 3—
Dissolve an appropriate quantity of USP Cefdinir Related Compound B RS in 0.1% Tetramethylammonium hydroxide solution to obtain a solution having a known concentration of about 0.04 mg per mL.
Resolution solution—
Transfer about 37.5 mg of accurately weighed USP Cefdinir RS into a 25-mL volumetric flask. Add about 10 mL of 0.1 M Phosphate buffer solution. Add 5.0 mL of each of Standard solution 2 and Standard solution 3, and dilute with 0.1% Tetramethylammonium hydroxide solution to volume.
Test solution—
Transfer an accurately weighed portion of the contents of 20 opened Capsules, equivalent to about 300 mg of Cefdinir, into a 200-mL volumetric flask. Dissolve in 30 mL of 0.1 M Phosphate buffer solution (Solution C), and dilute with 0.1% Tetramethylammonium hydroxide solution to volume to obtain a solution having a known concentration of about 1.5 mg per mL of cefdinir.
Chromatographic system
(see Chromatography  621
621 )—The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 150-mm column that contains 5-µm packing L1. The temperature of the Test solution is maintained at 4 ± 3
)—The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 150-mm column that contains 5-µm packing L1. The temperature of the Test solution is maintained at 4 ± 3 , and the column temperature is maintained at 40 ± 0.5
, and the column temperature is maintained at 40 ± 0.5 . The flow rate is about 1 mL per minute. The chromatograph is programmed as follows for a run time of about 60 minutes.
. The flow rate is about 1 mL per minute. The chromatograph is programmed as follows for a run time of about 60 minutes.
| Time (minutes) |
Mobile phase A
(%) |
Mobile phase B
(%) |
|
| 0–2 | 95 | 5 | isocratic |
| 2–22 | 95®75 | 5®25 | linear gradient |
| 22–32 | 75®50 | 25®50 | linear gradient |
| 32–37 | 50 | 50 | isocratic |
| 37–38 | 50®95 | 50®5 | linear gradient |
| 38–58 | 95 | 5 | isocratic |
Procedure—
Separately inject equal volumes (about 10 µL) of the Resolution solution, Standard solution 1, and the Test solution into the chromatograph, record the chromatograms, and measure the peak responses: the resolution between the cefdinir peak and the third peak of cefdinir related compound A (lactam ring cleavage lactones) is not less than 1.5; the tailing factor of cefdinir related compound B is not more than 1.5; and the relative standard deviation for the cefdinir peak response in replicate injections of Standard solution 1 is not more than 2.0%. The approximate relative retention times and the relative response factors for each of the related substances compared to cefdinir are given in Table 1. Calculate the percentage of each individual specified and unspecified impurity in the portion of Capsules taken, using the formula:
(100/F)(CS / CU)(rU / rS)
in which CS is the concentration, in mg per mL, of USP Cefdinir RS in Standard solution 1; CU is the concentration, in mg per mL, of cefdinir in the Test solution; rU is the peak response of each impurity obtained from the Test solution; rS is the peak response of cefdinir obtained from Standard solution 1; and F is the relative response factor as mentioned in Table 1 for each impurity. The specified and unspecified impurities meet the limits specified in Table 1.
Table 1
| Related Substance | Approximate Relative Retention Time |
Relative Response Factor |
Limit of Quantitation (% of Cefdinir) |
Limit (%) |
| Impurity VIII | 0.10 | 1.1 | 0.1 | NMT 0.5 |
| Impurity IV | 0.13 | 1.1 | 0.1 | NMT 0.5 |
| Impurity XIV | 0.36 | 1.0 | 0.05 | NMT 0.2 |
| Impurity V | 0.46 | 1.5 | 0.05 | NMT 0.7 |
| Impurity B3 | 0.77 | 1.0 | 0.05 | NMT 0.3 |
| Impurity XI | 0.75 | 1.0 | 0.05 | NMT 0.7 |
| Cefdinir related compound A (Lactam ring cleavage lactones-a)1 |
0.85 | 1.5 | 0.1 | NMT 2.5 |
| Cefdinir related compound A (Lactam ring cleavage lactones-b)1 |
0.94 | 1.5 | 0.1 | — |
| Cefdinir related compound A (Lactam ring cleavage lactones-c)1 |
1.11 | 1.5 | 0.1 | — |
| Cefdinir related compound A (Lactam ring cleavage lactones-d)1 |
1.14 | 1.5 | 0.1 | — |
| Impurity VI | 1.18 | 1.1 | 0.05 | NMT 0.2 |
| Impurity I | 1.23 | 1.2 | 0.05 | NMT 1.0 |
| Cefdinir related compound B1 | 1.28 | 1.1 | 0.05 | NMT 0.2 |
| Impurity XIII | 1.37 | 1.4 | 0.05 | NMT 0.5 |
| Impurity E3 | 1.44 | 1.0 | 0.05 | NMT 0.5 |
| Impurity XV | 1.49 | 1.0 | 0.05 | NMT 0.2 |
| Impurity VII | 1.51 | 1.1 | 0.05 | NMT 0.7 |
| Impurity IIIa2 | 1.62 | 1.3 | 0.05 | NMT 1.0 |
| Impurity IIIb2 | 1.64 | 1.3 | 0.05 | — |
| Impurity D3 | 1.82 | 1.0 | 0.05 | NMT 0.2 |
| Individual unidentified impurities | N/A | 1.0 | N/A | NMT 0.2 |
| Total unidentified impurities4 | N/A | N/A | N/A | NMT 1.0 |
| Total impurities | N/A | N/A | N/A | NMT 5.0 |
|
1
RS II is a mixture of 4 isomers designated as RS IIa, RS IIb, RS IIc, and RS IId. The sum of all values is reported and the total limit for all 4 isomers combined is 2.5%.
2
RS III is a mixture of 2 isomers designated as RS IIIa and RS IIIb. The sum of both values is reported and the total limit for both isomers combined is 1.0%.
3
Impurity B, Impurity D, and Impurity E are unidentified impurities.
4
The total unidentified impurities limit includes the % total of unidentified impurities B, D, and E and any other individual unidentified impurities.
|
||||
Assay—
0.1 M Phosphate buffer solution—
Dissolve 10.65 g of dibasic sodium phosphate and 3.40 g of monobasic potassium phosphate in 750 mL of water. Adjust with phosphoric acid or sodium hydroxide to a pH of 7.0 ± 0.05, and dilute with water to 1000 mL.
Citric acid buffer solution—
Dissolve 7.0 g of citric acid monohydrate in 1000 mL of water, and adjust with phosphoric acid to a pH of 2.0 ± 0.05.
Mobile phase—
Mix 1000 mL of Citric acid buffer solution with 111 mL of methanol and 28 mL of tetrahydrofuran. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard preparation—
Accurately weigh a known amount of USP Cefdinir RS into a suitable volumetric flask. Dissolve in 0.1 M Phosphate buffer solution to obtain a solution having a known concentration of about 0.05 mg per mL of cefdinir.
Assay preparation—
Transfer an accurately weighed portion of the contents of 20 opened Capsules, equivalent to about 100 mg of cefdinir, into a suitable volumetric flask. Dissolve in and dilute with 0.1 M Phosphate buffer solution to obtain a solution having a known concentration of about 0.05 mg per mL of cefdinir.
Resolution solution—
Accurately weigh a known amount of USP Cefdinir RS and m-hydroxybenzoic acid into a suitable volumetric flask. Dissolve in 0.1 M Phosphate buffer solution to obtain a solution having a known concentration of about 0.05 mg per mL of cefdinir and about 0.175 mg per mL of m-hydroxybenzoic acid.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 254-nm detector and a 3.9-mm × 150-mm column that contains 4-µm packing L1. The flow rate is maintained at about 1.4 mL per minute. Chromatograph the Resolution solution, and record the peak area response as directed for Procedure. The resolution between cefdinir and m-hydroxybenzoic acid is greater than 3.0; the tailing factor of the cefdinir peak is not more than 2.0; and the relative standard deviation for replicate injections of the Standard preparation is not more than 1.0%.
)—
The liquid chromatograph is equipped with a 254-nm detector and a 3.9-mm × 150-mm column that contains 4-µm packing L1. The flow rate is maintained at about 1.4 mL per minute. Chromatograph the Resolution solution, and record the peak area response as directed for Procedure. The resolution between cefdinir and m-hydroxybenzoic acid is greater than 3.0; the tailing factor of the cefdinir peak is not more than 2.0; and the relative standard deviation for replicate injections of the Standard preparation is not more than 1.0%.
Procedure—
Separately inject equal volumes (about 15 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the percentage of cefdinir (C14H13N5O5S2), based on the label claim, in the portion of Capsules taken by the formula:
 USP32
USP32
100(CS / CU)(rU / rS)
in which C S is the concentration, in mg per mL, of cefdinir in the Standard solution; CU is the concentration of cefdinir in the Assay preparation; and rU and rS are the peak responses of cefdinir in the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Ahalya Wise, M.S.
Scientist 1-301-816-8161 |
(MDANT05) Monograph Development-Antibiotics |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
|
| Margareth R.C. Marques, Ph.D.
Senior Scientist 1-301-816-8106 |
(BPC05) Biopharmaceutics05 |
USP32–NF27 Page 1827
Pharmacopeial Forum: Volume No. 34(1) Page 77