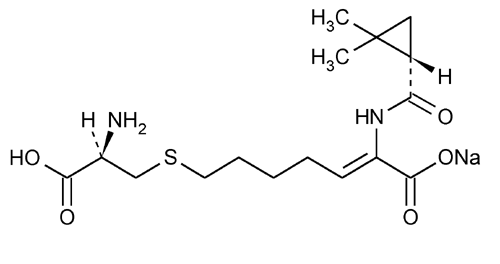
Cilastatin Sodium
2-Heptenoic acid, 7-[(2-amino-2-carboxyethyl)thio]-2-[[(2,2-dimethylcyclopropyl)carbonyl]amino]-, monosodium salt, [R-[R*,S*-(Z)]]-.
Sodium (Z)-7-[[(R)-2-amino-2-carboxyethyl]thio]-2-[(S)-2,2-dimethylcyclopropanecarboxamido]-2-heptenoate
» Cilastatin Sodium contains not less than 98.0 percent and not more than 101.5 percent of C16H25N2NaO5S, calculated on the anhydrous and solvent-free basis.
Packaging and storage—
Preserve in Containers for Sterile Solids as described under Injections  1
1 , and store in a cold place.
, and store in a cold place.
Labeling—
Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile.
Identification—
A:
The retention time of the major peak for cilastatin in the chromatogram of the Test solution, as obtained in the test for Chromatographic purity, corresponds to that in the chromatogram of a similar preparation of USP Cilastatin Ammonium Salt RS.
B:
Ignite a small portion of it on a platinum wire in a nonluminous flame: an intense yellow color is imparted to the flame.
Specific rotation  781S
781S :
between +41.5
:
between +41.5 and +44.5
and +44.5 , on the anhydrous and solvent-free basis.
, on the anhydrous and solvent-free basis.
Test solution:
10 mg per mL, in a mixture of methanol and hydrochloric acid (120:1).
Bacterial endotoxins  85
85 —
Where the label states that Cilastatin Sodium is sterile, it contains not more than 0.17 USP Endotoxin Unit per mg of cilastatin.
—
Where the label states that Cilastatin Sodium is sterile, it contains not more than 0.17 USP Endotoxin Unit per mg of cilastatin.
Sterility  71
71 —
Where the label states that Cilastatin Sodium is sterile, it meets the requirements when tested as directed for Membrane Filtration under Test for Sterility of the Product to be Examined, 6 g of specimen dissolved in 200 mL of Fluid A being used.
—
Where the label states that Cilastatin Sodium is sterile, it meets the requirements when tested as directed for Membrane Filtration under Test for Sterility of the Product to be Examined, 6 g of specimen dissolved in 200 mL of Fluid A being used.
pH  791
791 :
between 6.5 and 7.5, in a solution (1 in 100).
:
between 6.5 and 7.5, in a solution (1 in 100).
Water, Method I  921
921 :
not more than 2.0%.
:
not more than 2.0%.
Heavy metals, Method II  231
231 :
0.002%.
:
0.002%.
Limit of solvents—
Internal standard solution—
Transfer 0.5 mL of n-propyl alcohol to a 1000-mL volumetric flask, dilute with water to volume, and mix.
Standard solution—
Transfer 2.0 mL of acetone, 0.50 mL of methanol, and 0.50 mL of mesityl oxide to a 1000-mL volumetric flask, dilute with water to volume, and mix. Transfer 2.0 mL of this solution and 2.0 mL of Internal standard solution to a 10-mL volumetric flask, dilute with water to volume, and mix. This solution contains 316 µg of acetone, 79 µg of methanol, and 86 µg of mesityl oxide per mL.
Test solution—
Transfer about 200 mg of Cilastatin Sodium, accurately weighed, to a 10-mL volumetric flask, add 2.0 mL of Internal standard solution and about 5 mL of water, and dissolve by shaking. Dilute with water to volume, and mix.
Chromatographic system
(see Chromatography  621
621 )—The gas chromatograph is equipped with a flame-ionization detector and a 0.53-mm × 30-m capillary column, the internal wall of which is coated with a 1.0-µm film of liquid phase G16. The column temperature is maintained at 50
)—The gas chromatograph is equipped with a flame-ionization detector and a 0.53-mm × 30-m capillary column, the internal wall of which is coated with a 1.0-µm film of liquid phase G16. The column temperature is maintained at 50 for 2.5 minutes, then increased at a rate of 8
for 2.5 minutes, then increased at a rate of 8 per minute to 70
per minute to 70 , and maintained at 70
, and maintained at 70 for 0.5 minute; the injection port temperature is maintained at 160
for 0.5 minute; the injection port temperature is maintained at 160 ; the detector temperature is maintained at 250
; the detector temperature is maintained at 250 ; and helium is used as the carrier gas at a flow rate of about 9 mL per minute. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.26 for acetone, 0.35 for methanol, 0.67 for n-propyl alcohol, and 1.0 for mesityl oxide; and the relative standard deviation for replicate injections, determined from peak area ratios of each analyte to n-propyl alcohol, is not more than 5.0%.
; and helium is used as the carrier gas at a flow rate of about 9 mL per minute. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.26 for acetone, 0.35 for methanol, 0.67 for n-propyl alcohol, and 1.0 for mesityl oxide; and the relative standard deviation for replicate injections, determined from peak area ratios of each analyte to n-propyl alcohol, is not more than 5.0%.
Procedure—
Separately inject equal volumes (about 1 µL) of the Standard solution and the Test solution into the chromatograph, using the solvent (water) flush technique; record the chromatograms; and measure the areas for the acetone, methanol, n-propyl alcohol, and mesityl oxide peaks. Calculate the percentages of acetone, methanol, and mesityl oxide in the portion of Cilastatin Sodium taken by the formula:
(C/W)(RU / RS)
in which C is the concentration, in µg per mL, of the appropriate analyte in the Standard solution; W is the quantity, in mg, of Cilastatin Sodium taken to prepare the Test solution; and RU and RS are the peak area ratios of the corresponding analyte to n-propyl alcohol obtained from the Test solution and the Standard solution, respectively. Not more than 1.0% of acetone is found; not more than 0.5% of methanol is found; and not more than 0.4% of mesityl oxide is found.
Chromatographic purity—
Solvent—
Use water.
Solution A—
Prepare a mixture of dilute phosphoric acid (1 in 1000) and acetonitrile (700:300), pass through a filter having a 0.5-µm or finer porosity, and degas.
Solution B—
Use dilute phosphoric acid (1 in 1000). Pass through a filter having a 0.5-µm or finer porosity, and degas.
Mobile phase—
Use variable mixtures of Solution A and Solution B as directed for Chromatographic system. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Test solution—
Prepare a solution of Cilastatin Sodium in Solvent having a concentration of about 1.6 mg per mL.
Chromatographic system
(see Chromatography  621
621 )—The liquid chromatograph is equipped with a 210-nm detector and a 4.5-mm × 25-cm column containing packing L1. The column is maintained at a constant temperature of about 50
)—The liquid chromatograph is equipped with a 210-nm detector and a 4.5-mm × 25-cm column containing packing L1. The column is maintained at a constant temperature of about 50 . The flow rate is about 2 mL per minute. The chromatograph is programmed as follows.
. The flow rate is about 2 mL per minute. The chromatograph is programmed as follows.
Chromatograph the Test solution, and measure the peak responses as directed for Procedure: the capacity factor, k¢, is not less than 10; the column efficiency determined from the cilastatin peak is not less than 3000 theoretical plates; and the tailing factor is not more than 4.5.
| Time (minutes) |
Solution A
(%) |
Solution B
(%) |
Elution |
| 0 | 15 | 85 | equilibration |
| 0–30 | 15®100 | 85®0 | linear gradient |
Procedure—
Separately inject equal volumes (about 20 µL) of the Test solution and the Solvent into the chromatograph, record the chromatograms, and measure the areas of the peaks. Calculate the chromatographic purity, in percentage, of the portion of Cilastatin Sodium taken by the formula:
100rC / (rT  rB
rB  rA)
rA)
in which rC is the area of the cilastatin peak obtained from the Test solution; rT is the sum of the areas of all the peaks obtained from the Test solution; rB is the sum of the areas of all the peaks obtained from the Solvent; and rA is the response of the peak, if any, of nonretained substances, such as acetone, at the solvent front obtained from the Test solution: not less than 98.5% is found. Calculate the percentage of each impurity in the portion of Cilastatin Sodium taken by the formula:
100ri / (rT  rB
rB  rA)
rA)
in which ri is the peak area for each impurity in the chromatogram obtained from the Test solution and the other terms are as defined above: not more than 0.5% of any individual impurity is found.
Assay—
Transfer about 300 mg of Cilastatin Sodium, accurately weighed, to a suitable beaker, add 30 mL of methanol, and dissolve by swirling. Add 5 mL of water, and titrate potentiometrically with 0.1 N hydrochloric acid to a pH of about 3. Then titrate with 0.1 N sodium hydroxide until three inflection points have been observed. Calculate the titer difference, in mL, between the first and third inflection points. Each mL of 0.1 N sodium hydroxide is equivalent to 19.022 mg of C16H25N2NaO5S.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Ahalya Wise, M.S.
Scientist 1-301-816-8161 |
(MDANT05) Monograph Development-Antibiotics |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance | |
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance |
USP32–NF27 Page 1932
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.