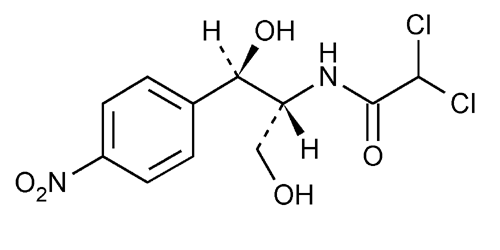
Chloramphenicol
Acetamide, 2,2-dichloro-N-[2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl]-, [R-(R*,R*)]-.
d-threo-(
» Chloramphenicol contains not less than 97.0 percent and not more than 103.0 percent of C11H12Cl2N2O5.
Packaging and storage—
Preserve in tight containers.
Labeling—
Where it is intended for use in preparing injectable or other sterile dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable or other sterile dosage forms.
Identification—
B:
The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
Specific rotation  781S
781S :
between +17.0
:
between +17.0 and +20.0
and +20.0 .
.
Test solution:
50 mg, undried, per mL, in dehydrated alcohol.
Crystallinity  695
695 :
meets the requirements.
:
meets the requirements.
Bacterial endotoxins  85
85 —
Where Chloramphenicol is intended for use in preparing injectable dosage forms, it contains not more than 0.2 USP Endotoxin Unit per mg of chloramphenicol.
—
Where Chloramphenicol is intended for use in preparing injectable dosage forms, it contains not more than 0.2 USP Endotoxin Unit per mg of chloramphenicol.
Sterility  71
71 —
Where the label states that Chloramphenicol is sterile, it meets the requirements when tested as directed for Membrane Filtration under Test for Sterility of the Product to be Examined, except to use 1 g of solid specimen.
—
Where the label states that Chloramphenicol is sterile, it meets the requirements when tested as directed for Membrane Filtration under Test for Sterility of the Product to be Examined, except to use 1 g of solid specimen.
pH  791
791 :
between 4.5 and 7.5, in an aqueous suspension containing 25 mg per mL.
:
between 4.5 and 7.5, in an aqueous suspension containing 25 mg per mL.
Chromatographic purity—
Dissolve an accurately weighed quantity of Chloramphenicol in methanol to obtain a test solution containing 10 mg per mL. Prepare a solution of USP Chloramphenicol RS in methanol containing 10 mg per mL (Standard solution A). Dilute portions of Standard solution A quantitatively with methanol to obtain Standard solution B containing 100 µg per mL and Standard solution C containing 50 µg per mL. Apply separate 20-µL portions of the test solution and Standard solutions B and C to a suitable thin-layer chromatographic plate (see Chromatography  621
621 ), coated with a 0.25-mm layer of chromatographic silica gel mixture. Develop the chromatogram in a solvent system consisting of a mixture of chloroform, methanol, and glacial acetic acid (79:14:7) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the chamber, air-dry, and examine under short-wavelength UV light: any spot other than the principal spot obtained from the test solution does not exceed in size or intensity the principal spot obtained from Standard solution B (1%), and the sum of the impurities represented by all of the spots other than the principal spot, based on a comparison of the intensities of such spots with the intensities of the principal spots obtained from Standard solutions B and C, does not exceed 2%.
), coated with a 0.25-mm layer of chromatographic silica gel mixture. Develop the chromatogram in a solvent system consisting of a mixture of chloroform, methanol, and glacial acetic acid (79:14:7) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the chamber, air-dry, and examine under short-wavelength UV light: any spot other than the principal spot obtained from the test solution does not exceed in size or intensity the principal spot obtained from Standard solution B (1%), and the sum of the impurities represented by all of the spots other than the principal spot, based on a comparison of the intensities of such spots with the intensities of the principal spots obtained from Standard solutions B and C, does not exceed 2%.
Assay—
Mobile phase—
Prepare a suitable filtered mixture of water, methanol, and glacial acetic acid (55:45:0.1). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard preparation—
Dissolve an accurately weighed quantity of USP Chloramphenicol RS in Mobile phase, and dilute quantitatively, and stepwise if necessary, with Mobile phase to obtain a solution having a known concentration of about 80 µg per mL. Filter a portion of this solution through a 0.5-µm or finer porosity filter, and use the clear filtrate as the Standard preparation.
Assay preparation—
Transfer about 200 mg of Chloramphenicol, accurately weighed, to a 100-mL volumetric flask, add Mobile phase to volume, and mix. Transfer 4.0 mL of the resulting solution to a 100-mL volumetric flask, dilute with Mobile phase to volume, and mix. Filter a portion of this solution through a 0.5-µm or finer porosity filter, and use the clear filtrate as the Assay preparation.
Chromatographic system
(see Chromatography  621
621 )—The liquid chromatograph is equipped with a 280-nm detector and a 4.6-mm × 10-cm column that contains 5-µm packing L1. The flow rate is about 1 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed under Procedure: the column efficiency determined from the analyte peak is not less than 1800 theoretical plates, the tailing factor is not more than 2.0, and the relative standard deviation for replicate injections is not more than 1.0%.
)—The liquid chromatograph is equipped with a 280-nm detector and a 4.6-mm × 10-cm column that contains 5-µm packing L1. The flow rate is about 1 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed under Procedure: the column efficiency determined from the analyte peak is not less than 1800 theoretical plates, the tailing factor is not more than 2.0, and the relative standard deviation for replicate injections is not more than 1.0%.
Procedure—
[note—Use peak heights where peak responses are indicated.] Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of C11H12Cl2N2O5 in the portion of Chloramphenicol taken by the formula:
2.5C(rU / rS)
in which C is the concentration, in µg per mL, of USP Chloramphenicol RS in the Standard preparation, and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Ahalya Wise, M.S.
Scientist 1-301-816-8161 |
(MDANT05) Monograph Development-Antibiotics |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance | |
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance |
USP32–NF27 Page 1885
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.