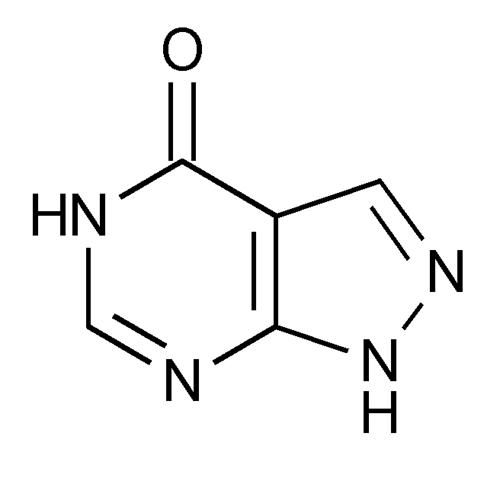
Allopurinol
» Allopurinol contains not less than 98.0 percent and not more than 102.0 percent of C5H4N4O, calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers. Store at room temperature.
USP Reference standards  11
11 —
—
USP Allopurinol RS.
USP Allopurinol Related Compound A RS .
.
USP Allopurinol Related Compound B RS .
.
USP Allopurinol Related Compound C RS .
.
USP Allopurinol Related Compound D RS .
.
USP Allopurinol Related Compound E RS.
USP Allopurinol Related Compound F RS.
USP Allopurinol RS.
USP Allopurinol Related Compound A RS
 .
.
USP Allopurinol Related Compound B RS
 .
.
USP Allopurinol Related Compound C RS
 .
.
USP Allopurinol Related Compound D RS
 .
.
USP Allopurinol Related Compound E RS.
USP Allopurinol Related Compound F RS.
Loss on drying  731
731 —
Dry it in vacuum at 105
—
Dry it in vacuum at 105 for 5 hours: it loses not more than 0.5% of its weight.
for 5 hours: it loses not more than 0.5% of its weight.
Change to read:
Related compounds—
[note—Store and inject the Standard solution and the Test solution at 8
Solution A—
Dissolve 1.25 g of monobasic potassium phosphate in 1000 mL of water, filter, and degas.
Solution B—
Use methanol.
Mobile phase—
Use variable mixtures of Solution A and Solution B as directed for Chromatographic system. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Diluent—
Prepare a mixture of Solution A and Solution B (90:10).
Standard stock solution—
Accurately weigh about 5 mg each of USP Allopurinol RS, USP Allopurinol Related Compound A RS, USP Allopurinol Related Compound B RS, USP Allopurinol Related Compound C RS, USP Allopurinol Related Compound D RS,  and
and USP32 USP Allopurinol Related Compound E RS,
USP32 USP Allopurinol Related Compound E RS, 
 USP32 and transfer to a 100-mL volumetric flask. Add 2.0 mL of 0.1 N sodium hydroxide, promptly sonicate with swirling for not more than 1 minute to dissolve,
USP32 and transfer to a 100-mL volumetric flask. Add 2.0 mL of 0.1 N sodium hydroxide, promptly sonicate with swirling for not more than 1 minute to dissolve,  add the entire volume of Allopurinol related compound F solution to the flask, rinse the flask in which Allopurinol related compound F solution was prepared with a small amount of Diluent, and add the rinsings to the solution.
add the entire volume of Allopurinol related compound F solution to the flask, rinse the flask in which Allopurinol related compound F solution was prepared with a small amount of Diluent, and add the rinsings to the solution. USP32 Sonicate for an additional 5 minutes. Dilute with Diluent to volume. [Note—This solution is stable for 48 hours when stored at 8
USP32 Sonicate for an additional 5 minutes. Dilute with Diluent to volume. [Note—This solution is stable for 48 hours when stored at 8 .]
.]
Standard solution—
Quantitatively dilute an accurately measured volume of the Standard stock solution with Diluent to obtain a solution having known concentrations of about 0.5 µg of allopurinol and about 0.5 µg each of allopurinol related compounds A, B C, D, E, and F per mL.
Test solution—
Transfer about 25 mg of Allopurinol, accurately weighed, to a 100-mL volumetric flask, add 5.0 mL of 0.1 N sodium hydroxide to dissolve, promptly sonicate with swirling for not more than 1 minute, add 80 mL of Diluent, and sonicate for an additional 5 minutes. Dilute with Diluent to volume.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 220-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L1. The column temperature is maintained at 30
)—
The liquid chromatograph is equipped with a 220-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L1. The column temperature is maintained at 30 . The flow rate is about 1.0 mL per minute. The chromatograph is programmed as follows.
. The flow rate is about 1.0 mL per minute. The chromatograph is programmed as follows.
Chromatograph the Standard solution, identify the peaks (see Table 1), and record the peak responses as directed for Procedure: the resolution, R, between allopurinol related compounds C and B is not less than 0.8; and the tailing factor for the allopurinol peak is not more than 1.5.
| Time (minutes) |
Solution A
(%) |
Solution B
(%) |
|
| 0–30 | 90®70 | 10®30 | linear gradient |
| 30–35 | 70 | 30 | isocratic |
| 35–36 | 70®90 | 30®10 | linear gradient |
| 36–46 | 90 | 10 | re-equilibration |
Procedure—
Separately inject equal volumes (about 40 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and identify the allopurinol peak and the peaks due to impurities listed in Table 1.
Table 1
| Relative Retention Time |
Limit (%) |
|
| Allopurinol related compound A1 | 0.62 | 0.2 |
| Allopurinol related compound C2 | 0.79 | 0.2 |
| Allopurinol related compound B3 | 0.81 | 0.2 |
| Allopurinol | 1.0 | — |
| Allopurinol related compound D4 | 4.4 | 0.2 |
| Allopurinol related compound E5 | 4.8 | 0.2 |
| Allopurinol related compound F6 | 6.5 | 0.2 |
|
1
3-Amino-1H-pyrazole-4-carboxamide.
|
||
|
2
5-(4H-1,2,4-Triazol-4-yl)-1H-pyrazole-4-carboxamide.
|
||
|
3
5-(Formylamino)-1H-pyrazole-4-carboxamide.
|
||
|
4
Ethyl-5-amino-1H-pyrazole-4-carboxylate.
|
||
|
5
Ethyl-5-(formylamino)-1H-pyrazole-4-carboxylate.
|
||
|
6
Ethyl-(E/Z)-3-(2-carbethoxy-2-cyanoethenyl)amino-1H-pyrazole-4-carboxylate.
|
||
Calculate the percentage of each impurity in the portion of Allopurinol taken by the formula:
10(C/W)(ri / rS)
in which C is the concentration, in µg per mL, of each individual impurity in the Standard solution; W is the weight, in mg, of Allopurinol taken to prepare the Test solution; and ri and rS are the peak responses for each individual impurity obtained from the Test solution and the Standard solution, respectively. [Note—For unspecified impurities, rS is the peak response for the allopurinol peak obtained from the Standard solution.] In addition to not exceeding the limits for each impurity in Table 1, not more than 0.1% of any individual unspecified impurity is found; and not more than 1.0% of total impurities is found.
Add the following:
Sodium hydroxide 2N solution—
Dissolve 8.5 g of sodium hydroxide in water, and dilute with the same solvent to 100 mL. Alternatively, a commercially available sodium hydroxide 2N solution can be used.
Diluent—
Prepare a mixture of Sodium hydroxide 2N solution and methanol (1:1).
Benzaldehyde solution—
Prepare a solution in Diluent containing 40 mg of benzaldehyde per mL. Prepare immediately before use.
Hydrazine solution—
Transfer 10.0 mg of hydrazine sulfate to a 50-mL volumetric flask, dissolve in Diluent by sonicating for about 2 minutes, and dilute with Diluent to volume. Dilute this solution quantitatively, and stepwise if necessary, with Diluent to obtain a solution having a known concentration of about 2.0 µg of hydrazine sulfate per mL.
Standard solution—
Transfer 5.0 mL of the Hydrazine solution to a suitable flask, add 4 mL of Benzaldehyde solution, mix, and allow to stand for 2.5 hours at room temperature. Add 5.0 mL of hexane, and shake for 1 minute. Allow the layers to separate, and use the upper (hexane) layer.
Test solution—
Transfer about 250.0 mg of Allopurinol, accurately weighed, to a suitable flask, and dissolve in 5.0 mL of Diluent. Add 4 mL of Benzaldehyde solution, mix, and allow to stand for 2.5 hours at room temperature. Add 5.0 mL of hexane, and shake for 1 minute. Allow the layers to separate, and use the upper (hexane) layer.
Blank solution
—Mix 5.0 mL of Diluent and 4 mL of Benzaldehyde solution, and allow to stand for 2.5 hours at room temperature. Add 5.0 mL of hexane, and shake for 1 minute. Allow the layers to separate, and use the upper (hexane) layer.
Mobile phase—
Prepare a mixture of hexane and isopropyl alcohol (95:5).
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 310-nm detector and a 4.0-mm × 25-cm column that contains 5-µm packing L10. The column temperature is maintained at 30
)—
The liquid chromatograph is equipped with a 310-nm detector and a 4.0-mm × 25-cm column that contains 5-µm packing L10. The column temperature is maintained at 30 , and the flow rate is about 1.5 mL per minute. Chromatograph the Standard solution, identify the components on the basis of their relative retention time (1.0 for benzaldehyde and about 0.8 for benzalazine), and record the peak responses as directed for Procedure: the resolution, R, between benzaldehyde and benzalazine is not less than 2.0; and the relative standard deviation for replicate injections is not greater than 15.0% for the benzalazine peak.
, and the flow rate is about 1.5 mL per minute. Chromatograph the Standard solution, identify the components on the basis of their relative retention time (1.0 for benzaldehyde and about 0.8 for benzalazine), and record the peak responses as directed for Procedure: the resolution, R, between benzaldehyde and benzalazine is not less than 2.0; and the relative standard deviation for replicate injections is not greater than 15.0% for the benzalazine peak.
Procedure—
Separately inject equal volumes (about 20 µL) of the Blank solution, the Standard solution, and the Test solution into a chromatograph, record the chromatogram for at least two times the retention time of the benzaldehyde peak, identify the components, and measure the responses of peaks due to benzalazine. Calculate the amount, in ppm, of hydrazine in the portion of Allopurinol taken by the formula:
 USP32
USP32
1000(32.05 / 130.12) (CS / CT)(rU / rS)
in which 32.05 and 130.12 are the molecular weights of hydrazine and hydrazine sulfate, respectively; 1000 is the conversion coefficient from µg per mL to ppm; CS is the concentration, in µg per mL, of hydrazine sulfate in the Hydrazine solution; CT is the concentration, in mg per mL, of allopurinol in the Allopurinol solution; and rU and rS are the peak responses for the benzalazine peak obtained from the Test solution and the Standard solution, respectively: not more than 10 ppm of hydrazine is found.
Assay—
[note—Store and inject the System suitability solution, the Standard preparation, and the Assay preparation at 8 , using a cooled autosampler.]
, using a cooled autosampler.]
Mobile phase—
Dissolve 1.25 g of monobasic potassium phosphate in 1000 mL of water, filter, and degas.
System suitability solution—
Transfer accurately weighed quantities of USP Allopurinol RS, USP Allopurinol Related Compound B RS, and USP Allopurinol Related Compound C RS, each to a suitable volumetric flask, dissolve in a small volume of 0.1 N sodium hydroxide, and immediately and quantitatively dilute with Mobile phase to obtain solutions having known concentrations of about 0.05 mg per mL. Transfer 1.0 mL of each of these three solutions to a 100-mL volumetric flask, dilute with Mobile phase to volume, and mix.
Standard preparation—
Dissolve an accurately weighed quantity of USP Allopurinol RS in a small volume of 0.1 N sodium hydroxide, and immediately and quantitatively dilute with Mobile phase to obtain a solution having a known concentration of about 0.5 mg per mL. Quantitatively dilute an accurately measured volume of this solution with Mobile phase to obtain a solution having a known concentration of about 0.08 mg of allopurinol per mL.
Assay preparation—
Transfer about 50 mg of Allopurinol, accurately weighed, to a 100-mL volumetric flask, dissolve in 5.0 mL of 0.1 N sodium hydroxide, immediately dilute with Mobile phase to volume, and mix. Quantitatively dilute an accurately measured volume of this solution with Mobile phase to obtain a solution having a known concentration of about 0.08 mg of allopurinol per mL.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 230-nm detector and a 4.6-mm × 25-cm column that contains packing L1. The flow rate is about 1.8 mL per minute. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the resolution, R, between allopurinol related compound B and allopurinol related compound C is not less than 1.1, and that between allopurinol related compound C and allopurinol is not less than 6.0. [note—For the purpose of identification, the relative retention times are about 0.7 for allopurinol related compound B, 0.8 for allopurinol related compound C, and 1.0 for allopurinol.] Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 2.0%.
)—
The liquid chromatograph is equipped with a 230-nm detector and a 4.6-mm × 25-cm column that contains packing L1. The flow rate is about 1.8 mL per minute. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the resolution, R, between allopurinol related compound B and allopurinol related compound C is not less than 1.1, and that between allopurinol related compound C and allopurinol is not less than 6.0. [note—For the purpose of identification, the relative retention times are about 0.7 for allopurinol related compound B, 0.8 for allopurinol related compound C, and 1.0 for allopurinol.] Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 20 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the percentage of C5H4N4O in the portion of Allopurinol taken by the formula:
100(CS / CU)(rU / rS)
in which CU and CS are the concentrations, in mg per mL, of allopurinol in the Assay preparation and the Standard preparation, respectively; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Elena Gonikberg, Ph.D.
Senior Scientist 1-301-816-8251 |
(MDGRE05) Monograph Development-Gastrointestinal Renal and Endocrine |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 1450
Pharmacopeial Forum: Volume No. 34(1) Page 70
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.