This general information chapter is intended to provide general guidance concerning storing, distributing, and shipping of Pharmacopeial preparations. It describes procedures to maintain proper storage environments for individual articles and to ensure a preparation's integrity, including its appearance, until it reaches the user. There is no change to any applicable requirements under Current Good Manufacturing Practices, approved labeling, state laws governing pharmacies, the USP General Notices and Requirements, or monographs. The section Preservation, Packaging, Storage, and Labeling under General Notices and Requirements provides definitions for storage conditions. All equipment used for recording, monitoring, and maintaining temperatures and humidity conditions should be calibrated on a regular basis. This calibration should be based on NIST or international standards (see Monitoring Devices—Time, Temperature, and Humidity  1118
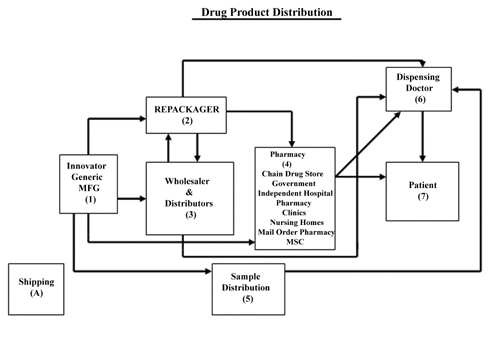
1118 ). A Pharmacopeial preparation may follow several potential routes from the original manufacturer to the patient. Figure 1 documents present-day routes and the associated risks. These risks include exposure to temperature excursions, humidity, light, and oxygen. For a discussion of climates, stability, and mean kinetic temperature, see Pharmaceutical Stability
). A Pharmacopeial preparation may follow several potential routes from the original manufacturer to the patient. Figure 1 documents present-day routes and the associated risks. These risks include exposure to temperature excursions, humidity, light, and oxygen. For a discussion of climates, stability, and mean kinetic temperature, see Pharmaceutical Stability  1150
1150 . Temperature- or humidity-sensitive articles are to be handled in accordance with General Notices.
. Temperature- or humidity-sensitive articles are to be handled in accordance with General Notices.
PACKAGING AND STORAGE STATEMENT IN MONOGRAPHS
Most articles have storage conditions identified by their labeling. Otherwise, it is expected that the conditions for storing the article are specified in the monograph according to definitions provided by the General Notices and Requirements in the section Storage Temperature, and Humidity under Preservation, Packaging, Storage, and Labeling. In cases where additional information on packaging and storage is desired, a specific statement can be provided in the Packaging and storage or the Labeling section of the individual monograph.
STORAGE IN WAREHOUSES, PHARMACIES, TRUCKS, SHIPPING DOCKS, AND OTHER LOCATIONS
Pharmacopeial articles are to be stored in locations that adhere to conditions established by the manufacturer. Where the desired conditions are not established, use storage conditions described in the General Notices and Requirements or in the applicable monograph.
Warehouses
Observation of the temperature variations in a warehouse should be made over a period of time to establish a meaningful temperature profile, including the temperature variations and conditions in the different parts of the warehouse. Such observations provide data and information as to where various products should and should not be stored.
establishing temperature profiles
Temperature profiles can be compiled by using a suitable number of thermometers or other temperature recording instruments. They should be placed throughout the warehouse in divided sections and should record the maximum and minimum temperatures during a 24-hour period for a total of three consecutive 24-hour periods. The following factors, some of which may give rise to extreme temperatures, should be considered during the process of temperature profiling: the size of the space, location of space heaters, sun-facing walls, low ceilings or roofs, and geographic location of the warehouse. Temperature profiling for warehouses already in use should be done at known times of external temperature extremes, e.g., for a period of not less than 3 hours when air temperatures are higher than 25 or less than 15
or less than 15 . Profiling should be conducted in both summer and winter. A mean kinetic temperature (MKT) should be obtained for any separate areas within the warehouse (see Pharmaceutical Calculations in Prescription Compounding
. Profiling should be conducted in both summer and winter. A mean kinetic temperature (MKT) should be obtained for any separate areas within the warehouse (see Pharmaceutical Calculations in Prescription Compounding  1160
1160 for samples of MKT calculations). The temperature profile report should provide recommendations for the use of each area and identification of any areas that are found unsuitable for storage of Pharmacopeial articles.
for samples of MKT calculations). The temperature profile report should provide recommendations for the use of each area and identification of any areas that are found unsuitable for storage of Pharmacopeial articles.
controlled room temperature
The General Notices provide a definition for Controlled Room Temperature. A temperature profiling study should demonstrate suitability for storing Pharmacopeial articles in areas determined to be at room or controlled room temperature. A suitable number of temperature and humidity recording instruments should be installed to record temperatures and to provide temperature and humidity profiles. Temperature recording should be conducted to meet the recommendations for establishing mean kinetic temperature and to comply with the warehouse's written procedures. These written procedures should have a reporting mechanism in place whereby a management tree is informed in the event that predefined high or low temperatures or humidity limits have been exceeded. Records can be reviewed as determined by the management system in accordance with established guidelines. Suitable training should be provided to persons who record temperatures, and proper quality accountability and tracking systems should be maintained.
storage at “cool,” “cold,” “refrigerator,” and “freezing” conditions
The General Notices provide definitions for cool, cold, refrigerator, and freezer temperatures. A temperature profiling study can be used to establish suitable areas for storing Pharmacopeial articles designated to be stored under these conditions. Equipment used for storing Pharmacopeial articles at these low temperatures should be qualified according to written procedures provided by the management system. Recording devices can be installed within the equipment and used to enable both air and product temperatures to be recorded at regular intervals. The number and location of monitoring devices should be determined based on the result of the temperature profile. Temperature records should be examined at least once every 24 hours or as provided in the equipment protocol. Cool or cold conditions are moisture-condensing conditions. Humidity-monitoring devices should be used in cases where the repackaged Pharmacopeial article is humidity-sensitive or labeled to avoid moisture. Additionally, there can be installed temperature-monitoring, and where necessary, humidity-monitoring alarm devices that have the capability of alerting personnel in the event that control is compromised. There should be protocols in place to address procedures for responding to failed temperature and humidity ranges both for normal working hours and outside normal working hours. Temperature and humidity should be reviewed at the times designated by the established protocol. The calibration and functioning of all temperature and humidity monitoring devices, including alarms and other associated equipment, should be checked on an annual or semiannual basis. Regular maintenance protocols should be in place for refrigeration equipment. There should be written agreements in place for all maintenance and evaluation procedures, and this may include an emergency situation protocol.
personnel training
Suitable training should be provided for personnel who handle Pharmacopeial articles with special storage temperature requirements. Personnel should know how to monitor temperatures and how to react to situations where adverse temperatures are identified. There should be written procedures in place such that the adverse temperatures are recorded and a report provided to the parties designated in the protocol.
qualification of “cold” equipment or stores
Only climate control equipment for which a contractor has provided documentation to assure its suitability for temperature and humidity requirements should be considered for use in cold storage. Qualification procedures on a regular basis should be independently conducted on equipment in cold stores to guarantee suitability and proper functioning. The procedure should demonstrate the temperature profile for both air and product temperatures when empty as well as when loaded. The procedure should also demonstrate the time taken for temperatures to exceed the maximum temperature in the event of a power failure. Qualification should consider thermal fluctuations that occur during stock replenishment and order removal. The results of the qualification should demonstrate the ability of the equipment to maintain the required temperature range in all areas, defining any zones which should not be used for storage such as those areas in close proximity to cooling coils, cold air streams from equipment ventilation, or doors. The variability of the system can be characterized by using the relative standard deviation. Thermal monitoring should establish that the system is rugged in that its temperature profile is consistent and reliable.
DISTRIBUTION AND SHIPMENT OF PHARMACOPEIAL ARTICLES
As indicated in Figure 1, a drug can take a variety of paths from the manufacturer to the patient. In the simplest form of the distribution system, the manufacturer ships directly to the customer, such as a doctor's office, clinic, or hospital. However, more often, the article leaves the manufacturer's chain of control and enters a complex system of handoffs that involve the distribution chain to the patient.
Shippers and distributors are to follow the proper storage and shipping requirements as indicated by the manufacturer. For particular cases, such as shipment of vaccines or other special care products, manufacturers may require special shipping and storage conditions generally referred to as “cold-chain management”. For example, manufacturers may attach temperature-monitoring devices and/or ship under specified controlled conditions to ensure that the desired temperature is maintained during distribution (see Monitoring Devices—Time, Temperature, and Humidity  1118
1118 ). Validated, available temperature- and/or humidity-monitoring technologies can be used to monitor the overall environmental effect on compendial articles during shipment and distribution. In these cases, the shipping conditions of the package are recorded. In general, extreme temperature conditions (i.e., excessive heat, freezing) should be avoided. Distribution systems chosen to deliver pharmaceutical products from the manufacturer to the consumer should take into account basic operational parameters, including timeliness and accountability. The manufacturer's FDA-approved storage conditions, printed in the labeling of the product, should be observed carefully at each destination of the distribution chain (see Figure 1), unless specifically instructed otherwise in the immediate label of a shipping container. This may be the case for certain pallet-sized shipping containers where the amount of refrigerant contained (e.g., dry ice, gel packs) is based on an anticipated exterior condition approximating controlled room temperature. In such cases, placing the shipping container in a refrigerator could lead to the product inside freezing, potentially affecting its quality. Items requiring special handling conditions will have those conditions clearly indicated in the labeling for the product. The Prescription Drug Marketing Act of 1987 and the ensuing regulations in 21 CFR Part 203, Prescription Drug Marketing, and Part 205, Guidelines for State Licensing of Wholesale Prescription Drug Distributors, provide the necessary regulations and guidance for several legs of the distribution chain for the prescription drug. The manufacturers and distributors should work together to establish proper distribution and product-handling requirements for the purpose of ensuring appropriate product maintenance in transit. Pharmacists and physicians should educate patients regarding proper storage of products to ensure product integrity at the patient level. Information that may be considered in determining the ability of pharmaceutical articles to maintain their Pharmacopeial requirements of identity, strength, quality, and purity through the distribution channel may include, but is not limited to the following: ICH stability studies, temperature cycling studies, stability shipping studies, ongoing regulatory stability commitment studies, market experience portfolio (i.e., product complaint files, historical product performance data, product development data), and product labeling commitments.
). Validated, available temperature- and/or humidity-monitoring technologies can be used to monitor the overall environmental effect on compendial articles during shipment and distribution. In these cases, the shipping conditions of the package are recorded. In general, extreme temperature conditions (i.e., excessive heat, freezing) should be avoided. Distribution systems chosen to deliver pharmaceutical products from the manufacturer to the consumer should take into account basic operational parameters, including timeliness and accountability. The manufacturer's FDA-approved storage conditions, printed in the labeling of the product, should be observed carefully at each destination of the distribution chain (see Figure 1), unless specifically instructed otherwise in the immediate label of a shipping container. This may be the case for certain pallet-sized shipping containers where the amount of refrigerant contained (e.g., dry ice, gel packs) is based on an anticipated exterior condition approximating controlled room temperature. In such cases, placing the shipping container in a refrigerator could lead to the product inside freezing, potentially affecting its quality. Items requiring special handling conditions will have those conditions clearly indicated in the labeling for the product. The Prescription Drug Marketing Act of 1987 and the ensuing regulations in 21 CFR Part 203, Prescription Drug Marketing, and Part 205, Guidelines for State Licensing of Wholesale Prescription Drug Distributors, provide the necessary regulations and guidance for several legs of the distribution chain for the prescription drug. The manufacturers and distributors should work together to establish proper distribution and product-handling requirements for the purpose of ensuring appropriate product maintenance in transit. Pharmacists and physicians should educate patients regarding proper storage of products to ensure product integrity at the patient level. Information that may be considered in determining the ability of pharmaceutical articles to maintain their Pharmacopeial requirements of identity, strength, quality, and purity through the distribution channel may include, but is not limited to the following: ICH stability studies, temperature cycling studies, stability shipping studies, ongoing regulatory stability commitment studies, market experience portfolio (i.e., product complaint files, historical product performance data, product development data), and product labeling commitments.
Qualification Protocol
Operational and performance testing should be parts of a formal qualification protocol that may use controlled environments or actual field testing based on the projected transportation channel. These should reflect actual load configurations, conditions, and expected environmental extremes. Temperature and humidity monitors should be placed into the product or a representative thereof. Testing consists of consecutive replicate field transportation tests using typical loads, according to an established protocol.
Physical Challenges
Most products are sufficiently robust to withstand distribution with minimal protection from routine, well-understood physical and environmental hazards. Several standard test methods are available for evaluating package performance factors under well-documented shock, vibration, and other transit elements. The American Society for Testing and Materials document, “Standard Practice for Performance Testing of Shipping Containers and Systems” (ASTM D4169-98), and the International Safe Transit Association's (ISTA) specifications have similar methods for evaluation of shipping performance for various types of transit modes such as less-than-truckload (LTL), small package, rail car, air freight, etc. From the manufacturer's perspective, these tests are very useful in evaluating the product and package durability and fragility. The tests are usually performed on shipping carton quantities of a specific stock keeping unit (SKU) as an unbroken whole. Fragility problems can be corrected with package modifications, which could include placing cotton or rayon coilers in bottles or placing top and bottom pads in the shipping case to reduce package breakage. Not all protective packaging elements follow the SKU through the system.
Basic packaging principles are observed when separating the contents of the manufacturer's shipping container or pallet load into smaller quantities or when shipping mixed product loads. For example, glass containers are wrapped in a bubble wrap or other shock-absorbent material, and the void spaces are filled with dunnage (e.g., foam “peanuts,” shredded or tightly crumpled paper, bubble wrap) to protect the contents from shifting and drop impact. Large-volume liquid containers may be bagged in plastic and kept isolated to prevent leakage to, or damage of, adjacent packages. “Skin packaging,” a term describing a heat-shrink film that anchors the load to fiberboard and prevents load shift, can be an excellent method of protecting some products, but it may be inappropriate for heat-sensitive products. The shipping carton should have correct Edge Crush Test (ECT) characteristics for freight being shipped according to Item 222 of the National Motor Freight Classification and Rule 41 of the Uniform Freight Classification.
Temperature Challenges
Shipping of temperature-sensitive articles requiring thermally controlled packaging presents a special challenge. Unlike shock, vibration, and other physical hazards, thermal hazards tend to be unique to a given system. Except for temperature-controlled trucks, the distribution environment is widely variable and depends upon a range of factors, including points of origin and destination, article and container sensitivities to cold, accidental freezing or heat, transit mode (e.g., air, truck, combination), time, weather or season, and carrier type (e.g., small package carrier or integrator, freight forwarder, U.S. Postal Service). The shippers should know and understand the systems they use and should design the protective package accordingly. Storage temperature ranges may not be indicative of the allowable tolerances during shipping. Articles labeled for special storage conditions (between 2 and 8
and 8 ) vary widely in their tolerance of short-term exposure to heat and cold. Some, such as soft gelatin capsules and suppositories, carry specific upper limits on both shipping containers and SKUs. A temperature cycling study intended to identify those articles affected by multiple, short-term excursions beyond the storage temperature limits should be performed. These data provide wholesalers and distributors with clearer identification of those drug products that may require special handling during particular climate conditions.
) vary widely in their tolerance of short-term exposure to heat and cold. Some, such as soft gelatin capsules and suppositories, carry specific upper limits on both shipping containers and SKUs. A temperature cycling study intended to identify those articles affected by multiple, short-term excursions beyond the storage temperature limits should be performed. These data provide wholesalers and distributors with clearer identification of those drug products that may require special handling during particular climate conditions.
Materials
Two commonly used types of refrigerant are dry ice (frozen carbon dioxide gas) and wet ice (frozen water), which appears as crushed ice or in various refrigerant packs containing water mixtures with specific freezing points. Phase-change materials are also available for specialized needs. Refrigerant packs should have the correct freezing point and be cooled to the proper surface temperature prior to use. Articles harmed by accidental freezing may require a barrier between the refrigerant and the product or some other special packaging. Insulating materials commonly available include foil laminates, bubble pack, corrugated, fabricated, and molded expanded polystyrene (EPS) cartons, and fabricated or molded urethane foam cartons, with or without additional interior components. Recognized standard test methods for evaluating insulated containers are currently limited to ASTM D3103-92, Standard Test Method for Thermal Insulation Quality of Packages and a method under development by ISTA. Neither one fully addresses all of the issues involved, but both include useful information on testing procedures. The tests should be modified based on the specific system adopted by the shipper. The manufacturer may be able to supply helpful data on specific articles and their requirements.
SPECIAL HANDLING
Certain classes of Pharmacopeial articles may require special handling. Such articles include products classified as dangerous goods under the Department of Transportation (DOT), state, local, or carrier rules; or products classified as controlled substances by the Drug Enforcement Administration (DEA) or by individual states.
Receipt of Pharmacopeial Articles
Upon arrival of Pharmacopeial articles to warehouse loading docks, premises, and other arrival areas, the Pharmacopeial articles are to be transferred to their manufacturer-designated storage environment within 2 hours of receipt. Limitation of the time spent in the uncontrolled environments of the loading dock is important to ensure that the integrity of the preparation is maintained. This is particularly important for temperature-sensitive items. The delivery document should be reviewed at receiving sites to ensure that the Pharmacopeial articles have not been subjected to any delays during shipment that could result in exposure of the article to extremes of temperature, or to any other extreme or undesirable conditions. In addition, to the extent possible, the receiving personnel should ensure that the ruggedness requirements in shipment have been met. For Pharmacopeial articles requiring extreme caution, special handling, or refrigerator temperature storage conditions, those who supply the articles (e.g., wholesalers and manufacturers) and delivery contractors should provide documented evidence to show that the required temperature range has been maintained during transportation. In the event that a deviation from the required temperature range has been observed during shipment of an article requiring such a shipping condition, the supplier or delivery contractors should document the temperature and the length of time the compendial article was not within the designated storage temperature. The pharmaceutical manufacturer may be contacted to determine the significance of unusual variances.
Distribution or Shipping Vehicles
Vehicles used for shipping or distribution of Pharmacopeial articles designated for storage at controlled room temperature should be suitably equipped to ensure that the temperature excursions encountered are within those allowed under the definition of controlled room temperature. Steps should be taken so that extremes of temperature, whether above or below the specified temperatures, should not be encountered during delivery procedures.
Vehicle Qualification
Where practical, suitable monitoring devices, as determined by the manufacturer and vehicle supplier, should be placed in different areas of the truck to establish a temperature profile of the truck over a 24-hour period during a hot summer day, average high, and a cold winter day, average low, and during a normal or typical day. The derived temperature of the different parts of the truck may be used to determine the location on the truck where Pharmacopeial articles can be stored appropriately during shipping (see Monitoring Devices—Time, Temperature, and Humidity  1118
1118 ).
).
Pharmaceutical Delivery Staff
As part of the contractual agreement between the delivery contractors and the manufacturers, the delivery staff should receive appropriate training to ensure that they are aware of the correct procedures to follow in maintaining products at the correct temperature. There may be written procedures that should be documented. In addition, the transportation personnel should have proper knowledge of the temperature profile of the vehicle to ensure proper placement of the Pharmacopeial articles in the vehicle. Pharmacopeial articles requiring special handling (e.g., refrigeration) or environmentally sensitive preparations should be transported in a suitably equipped vehicle to ensure that the articles are maintained at the correct temperature during distribution, shipping, and delivery and up to the point of receipt. Special arrangements should be made to inform receiving personnel, pharmacists, or other appropriate customers that the package includes articles with special storage and handling specifications and are to be transferred immediately to the appropriate storage location. The manufacturer, shipper, or delivery agency should provide appropriate evidence to show that the required temperature has been maintained throughout shipment and distribution.
SHIPMENT FROM MANUFACTURER TO WHOLESALER
Wholesaler
The wholesaler receiving the pharmaceutical articles should ensure that on arrival, the pharmaceutical articles are transferred to the correct environment without delay, as directed by the manufacturer, ideally within 2 hours of receipt. The wholesaler should examine the delivery documentation to ensure that the products have not been subjected to any delays during shipping and distribution that could result in products being exposed to extreme temperatures (see also the previous section, Pharmaceutical Delivery Staff, for staff expectations). The vehicles used for shipping of Pharmacopeial articles to the wholesaler, especially products requiring storage at low temperatures, should be suitably equipped to ensure that products are maintained at the correct temperature during shipping and distribution and up to the point of receipt. The receiving wholesaler staff should be informed that the articles are transferred to appropriate storage locations without delays. The vehicles used for shipping of Pharmacopeial articles requiring storage at room or controlled room temperatures should be suitably equipped to ensure that extremes of temperature, either above or below the specified temperature, do not occur during delivery procedures. Warehouse staff may receive appropriate training to ensure that the correct procedures are followed to maintain required temperature conditions (see Pharmaceutical Delivery Staff). Where necessary, a monitoring device for temperature and/or humidity should be used during shipping and distribution.
Compromised Temperature Conditions
A procedure should be in place in the warehouse to define the action that should be taken in the event of deviation from required storage conditions. Suitable records should be maintained to explain the reason for deviation and the resulting action that is taken. The product in question should then be placed in a quarantine status. Advice on the suitability of the product for use should be sought from the manufacturer or supplier of the product. The manufacturer's response should be documented prior to issuing the product to the customer, if that product is to be issued to the customer.
SHIPMENT FROM MANUFACTURER OR WHOLESALER TO PHARMACY
Pharmacy
The pharmacy receiving the pharmaceutical articles should ensure that on arrival, the pharmaceutical articles are transferred to the correct environment without delay, as directed by the manufacturer, ideally within 2 hours of receipt. The pharmacy personnel should examine the delivery documentation to ensure that the products have not been subjected to any delays during shipping and distribution, which could result in the products being exposed to extreme temperatures (see also the section, Pharmaceutical Delivery Staff, for staff expectations). The vehicles used for shipping of Pharmacopeial articles to the pharmacy, especially products requiring storage at low temperatures, should be suitably equipped to ensure that products are maintained at the correct temperature during shipping and distribution and up to the point of receipt. Receiving pharmacy staff should be informed that the articles are to be transferred to appropriate storage without delays. The vehicles used for shipping of Pharmacopeial articles requiring storage at room or controlled room temperatures should be suitably equipped to ensure that extremes of temperature, either above or below the specified temperature, do not occur during delivery procedures. Pharmacy staff may receive appropriate training to ensure that the correct procedures are followed to maintain required temperature conditions (see Pharmaceutical Delivery Staff). Where necessary, a monitoring device for temperature and/or humidity may be used during shipping and distribution.
Compromised Temperature Conditions
The pharmacy should maintain appropriate procedures to define action that should be taken in the event of deviation from the required storage conditions. Suitable records should be maintained to explain the reason for deviation and the resulting action taken (including whether the product is issued to the patient or customer). Advice on the suitability of the product for use as an acceptable drug article should be sought from the manufacturer or supplier of the product.
SHIPMENT FROM PHARMACY TO PATIENT OR CUSTOMER
The pharmacy should provide an appropriate label on the package sent through air or surface routes so that the deliverer does not place the package in a mailbox exposed to extremes in temperature. In the event that no one is available to receive the package, the deliverer should return the package to the post office or service office, and store it in a cool or air-conditioned area until the patient can receive the medication. In the event that the package has not been delivered for more than 2 days, the package may be returned to the pharmacy. For temperature-sensitive articles, it is important that proper arrangements be made to protect the drug from exposure to high temperatures, or in some cases, from freezing conditions. Such arrangements may include the following: insulating the packaging, or packaging with coolant included; overnight shipping; and pre-arranged pick-up. In such cases, the pharmacy should provide on the external package a statement of an acceptable period of delay for delivery. The patient or customer should examine the delivery documentation to ensure that the package has not been subjected to any unacceptable delays during shipping and distribution. The patient or customer receiving the pharmaceutical articles, either by mail, delivery vehicle from the pharmacy, or directly from the physician or pharmacy, should be advised that upon receipt the articles are to be transferred to appropriate storage conditions without delay, as directed by the pharmacy, ideally within 2 hours of receipt. The vehicle used for air or surface shipping and distribution of pharmaceutical packages to the patient or customer, especially those requiring low temperatures, should contain the article suitably packaged in containers that maintain the desired storage conditions until the article reaches the patient or customer. The vehicles used for shipping and distribution of pharmaceutical articles to patient or customer, especially those requiring storage at room or controlled room temperatures, should be suitably equipped during extreme temperature conditions such that the packages are not exposed to extremes of temperature either in winter or summer months. In the event that the vehicle is not adequately equipped with air conditioning or heating to protect the product, the time that the article is exposed to ambient conditions should be strictly limited, ideally not more than 2 hours. Where appropriate, a monitoring device may be used to ensure that required temperatures are maintained until the package reaches the patient or customer. If stability studies for the Pharmacopeial preparation indicate that it is particularly sensitive to environmental insults or if appropriate shipping safeguards described in this section are not feasible, then the preparation should be shipped by a different method whereby environmental control can be maintained.
Compromised Temperature Conditions
There should be appropriate procedures in the pharmacy that ships the article to the patient or customer defining the action that should be taken in the event that a patient reports that there has been a deviation from required storage conditions for an article, including any environmentally sensitive preparations, prior to the point of receipt. Advice on the suitability of the product for use should be provided to the patient or customer after the manufacturer or supplier's advice has been sought by the pharmacy. If the patient is advised to use the article, such advice should be documented and noted appropriately by the pharmacy. Otherwise, appropriate arrangements should be made to promptly replace the suspect article. For mail order items, replacement from local pharmacies may be an option to ensure an uninterrupted supply of medication.
RETURNS OF PHARMACEUTICAL ARTICLES FROM PATIENTS OR CUSTOMERS
The wholesaler, manufacturer, and pharmacy personnel should evaluate the validity of the request for return, and maintain an auditable account of the return receipt. For products in unopened manufacturer's containers that have been at variance during shipment, arrangement may be made to return the products to the manufacturer, wholesaler, or pharmacy preferably within 3 working days of receipt. The supplier may request records or written confirmation by the patient to show that the product was stored properly while in possession of the customer.
STORAGE OF PHYSICIAN SAMPLES HANDLED BY SALES REPRESENTATIVES IN AUTOMOBILES
Storage of physician samples by sales representatives is regulated under 21CFR 203.34(b)(4); each manufacturer or distributor is to have appropriate policies in place to ensure that proper storage is maintained. The following suggestions may be considered in response to this need and are of interest to practitioners who may observe actual practices. Automobile trunks or passenger cabins used for the storage and distribution of physician samples should be monitored to determine the temperature profile of the trunk or passenger cabin. Suitable monitoring devices as determined by the sales representative may be placed in different areas of the trunk or passenger cabin on a hot summer and a cold winter day. Measurements should also be made during typical 24-hour periods, and the derived temperature should be used for calculation of the mean kinetic temperature at which the sample is stored (see Pharmaceutical Calculations in Prescription Compounding  1160
1160 for examples of MKT calculations). If the Pharmacopeial article designated for storage requires storage at controlled room temperature, then suitable measures should be taken to maintain the sample within the allowable limits of the storage parameters. Environmentally-sensitive preparations should not be stored in automobile trunks or passenger cabins. Medications stored in automobile trunks or passenger cabins should be removed at the end of 3 days. Sales representatives should consider parking automobiles in shaded areas to avoid extreme heat during the summer and in garages to avoid freezing temperatures during the winter. The use of vouchers from the manufacturer that patients could use to obtain medication samples from participating pharmacies is an alternative way of providing drug samples.
for examples of MKT calculations). If the Pharmacopeial article designated for storage requires storage at controlled room temperature, then suitable measures should be taken to maintain the sample within the allowable limits of the storage parameters. Environmentally-sensitive preparations should not be stored in automobile trunks or passenger cabins. Medications stored in automobile trunks or passenger cabins should be removed at the end of 3 days. Sales representatives should consider parking automobiles in shaded areas to avoid extreme heat during the summer and in garages to avoid freezing temperatures during the winter. The use of vouchers from the manufacturer that patients could use to obtain medication samples from participating pharmacies is an alternative way of providing drug samples.
STABILITY, STORAGE, AND LABELING
The design of stability studies of Pharmacopeial articles is based on knowledge of the behavior, properties, and stability of the drug substance and experience gained from clinical formulation studies.1 The length of the studies and the storage conditions for a Pharmacopeial article should be sufficient to cover storage, shipment, distribution, and subsequent use of a Pharmacopeial article. The data gathered from ICH accelerated testing or from testing at an ICH intermediate condition may be used to evaluate the effect of short-term excursions outside the label storage conditions such as those that might occur during shipping. See Pharmaceutical Stability  1150
1150 .
.
STATEMENTS/LABELING OF THE IMMEDIATE CONTAINERS OR PACKAGE INSERT
Storage statements should be based on the stability evaluations of the Pharmacopeial drug substances and in accordance with national and international requirements.
Room Temperature Storage Statements—
For products with a storage statement reading, “Store at controlled room temperature,” the labeling should read as follows on the package insert: “Store at 20 C to 25
C to 25 C (68
C (68 F to 77
F to 77 F), excursions permitted between 15
F), excursions permitted between 15 C and 30
C and 30 C (between 59
C (between 59 F and 86
F and 86 F). Brief exposure to temperatures up to 40
F). Brief exposure to temperatures up to 40 C (104
C (104 F) may be tolerated provided the mean kinetic temperature does not exceed 25
F) may be tolerated provided the mean kinetic temperature does not exceed 25 C (77
C (77 F); however, such exposure should be minimized.”
F); however, such exposure should be minimized.”
On the immediate container label, the following may read for controlled room temperature (CRT): “Store at 20 C to 25
C to 25 C (68
C (68 F to 77
F to 77 F), excursions permitted between 15
F), excursions permitted between 15 C and 30
C and 30 C (between 59
C (between 59 F and 86
F and 86 F).”
F).”
Cool Storage Statement—
The storage statement for labeling may be as follows: “Store in a cool place, 8 C to 15
C to 15 C (46
C (46 F to 59
F to 59 F).”
F).”
Refrigerator Storage Statement—
The storage statement for labeling may be as follows: “Store in a refrigerator, 2 C to 8
C to 8 C (36
C (36 F to 46
F to 46 F).”
F).”
Freezer Storage Statement—
The storage statement for labeling may be as follows: “Store in a freezer, –25 C to –10
C to –10 C (–13
C (–13 F to 14
F to 14 F).”
F).”
See the General Notices for all other applicable storage conditions, such as Storage Under Nonspecific Conditions and store in a Dry Place. Additional cautionary statements to protect the Pharmacopeial drug product from extreme temperature and humidity conditions may be included on the container label and package insert, as the manufacturer desires.
1
See International Conference on Harmonization EWG Q1 A&B; see also FDA Guidance for Industry: Stability Testing of Drug Substances and Drug Products (www.fda.gov).
Auxiliary Information— Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| General Chapter | Desmond G. Hunt, Ph.D.
Scientist 1-301-816-8341 |
(PS05) Packaging and Storage 05 |
USP32–NF27 Page 536
Pharmacopeial Forum: Volume No. 32(4) Page 1208