- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Sorbitol |
 |
(Ph. Eur. monograph 0435)
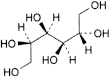
C6H14O6 182.2 50-70-4
Used for parenteral nutrition.
Ph Eur
d-Glucitol (d-sorbitol).
97.0 per cent to 102.0 per cent (anhydrous substance).
White or almost white, crystalline powder.
Very soluble in water, practically insoluble in ethanol (96 per cent).
It shows polymorphism (5.9).
First identification A.
Second identification B, C, D.
A. Examine the chromatograms obtained in the assay.
Results The principal peak in the chromatogram obtained with the test solution is similar in retention time and size to the principal peak in the chromatogram obtained with reference solution (a).
B. Dissolve 0.5 g with heating in a mixture of 0.5 mL of pyridine R and 5 mL of acetic anhydride R. After 10 min, pour the solution into 25 mL of water R and allow to stand in iced water for 2 h. The precipitate, recrystallised from a small volume of ethanol (96 per cent) R and dried in vacuo, melts (2.2.14) at 98 °C to 104 °C.
C. Thin-layer chromatography (2.2.27).
Test solution Dissolve 25 mg of the substance to be examined in water R and dilute to 10 mL with the same solvent.
Reference solution (a) Dissolve 25 mg of sorbitol CRS in water R and dilute to 10 mL with the same solvent.
Reference solution (b) Dissolve 25 mg of mannitol CRS and 25 mg of sorbitol CRS in water R and dilute to 10 mL with the same solvent.
Plate TLC silica gel G plate R.
Mobile phase water R, ethyl acetate R, propanol R (10:20:70 V/V/V).
Application 2 µL.
Development Over a path of 17 cm.
Drying In air.
Detection Spray with 4-aminobenzoic acid solution R; dry in a current of cold air until the acetone is removed; heat at 100 °C for 15 min; allow to cool and spray with a 2 g/L solution of sodium periodate R; dry in a current of cold air; heat at 100 °C for 15 min.
System suitability Reference solution (b):
- — the chromatogram shows 2 clearly separated spots.
Results The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with reference solution (a).
D. Specific optical rotation (2.2.7): + 4.0 to + 7.0 (anhydrous substance).
Dissolve 5.00 g of the substance to be examined and 6.4 g of disodium tetraborate R in 40 mL of water R. Allow to stand for 1 h, shaking occasionally, and dilute to 50.0 mL with water R. Filter if necessary.
The solution is clear (2.2.1) and colourless (2.2.2, Method II).
Dissolve 5 g in water R and dilute to 50 mL with the same solvent.
Maximum 20 µS·cm-1.
Dissolve 20.0 g in carbon dioxide-free water R prepared from distilled water R and dilute to 100.0 mL with the same solvent. Measure the conductivity of the solution while gently stirring with a magnetic stirrer.
Maximum 0.2 per cent, expressed as glucose equivalent.
Dissolve 5.0 g in 6 mL of water R with the aid of gentle heat. Cool and add 20 mL of cupri-citric solution R and a few glass beads. Heat so that boiling begins after 4 min and maintain boiling for 3 min. Cool rapidly and add 100 mL of a 2.4 per cent V/V solution of glacial acetic acid R and 20.0 mL of 0.025 M iodine. With continuous shaking, add 25 mL of a mixture of 6 volumes of hydrochloric acid R and 94 volumes of water R and, when the precipitate has dissolved, titrate the excess of iodine with 0.05 M sodium thiosulfate using 1 mL of starch solution R, added towards the end of the titration, as indicator. Not less than 12.8 mL of 0.05 M sodium thiosulfate is required.
Liquid chromatography (2.2.29).
Test solution Dissolve 5.0 g of the substance to be examined in 20 mL of water R and dilute to 100.0 mL with the same solvent.
Reference solution (a) Dissolve 0.50 g of sorbitol CRS in 2 mL of water R and dilute to 10.0 mL with the same solvent.
Reference solution (b) Dilute 2.0 mL of the test solution to 100.0 mL with water R.
Reference solution (c) Dilute 5.0 mL of reference solution (b) to 100.0 mL with water R.
Reference solution (d) Dissolve 0.5 g of sorbitol R and 0.5 g of mannitol R (impurity A) in 5 mL of water R and dilute to 10.0 mL with the same solvent.
- — size: l = 0.3 m, Ø = 7.8 mm;
- — stationary phase: strong cation exchange resin (calcium form) R (9 µm);
- — temperature: 85 ± 1 °C.
Mobile phase degassed water R.
Flow rate 0.5 mL/min.
Detection Refractometer maintained at a constant temperature.
Injection 20 µL of the test solution and reference solutions (b), (c) and (d).
Run time 3 times the retention time of sorbitol.
Relative retention With reference to sorbitol (retention time = about 27 min): impurity C = about 0.6; impurity A = about 0.8; impurity B = about 1.1.
System suitability Reference solution (d):
- — resolution: minimum 2 between the peaks due to impurity A and sorbitol.
- — any impurity: for each impurity, not more than the area of the principal peak in the romatogram obtained with reference solution (b) (2 per cent);
- — total: not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (3 per cent);
- — disregard limit: the area of the principal peak in the chromatogram obtained with reference solution (c) (0.1 per cent).
Maximum 0.5 ppm.
Maximum 1 ppm.
Dissolve the substance to be examined in 150.0 mL of the prescribed mixture of solvents.
Maximum 1.5 per cent, determined on 1.00 g.
If intended for use in the manufacture of parenteral preparations:
- — TAMC: acceptance criterion 102 CFU/g (2.6.12).
If not intended for use in the manufacture of parenteral preparations:
- — TAMC: acceptance criterion 103 CFU/g (2.6.12);
- — TYMC: acceptance criterion 102 CFU/g (2.6.12);
- — absence of Escherichia coli (2.6.13);
- — absence of Salmonella (2.6.13).
If intended for use in the manufacture of parenteral preparations without a further appropriate procedure for the removal of bacterial endotoxins:
- — less than 4 IU/g for parenteral preparations having a concentration of less than 100 g/L of sorbitol;
- — less than 2.5 IU/g for parenteral preparations having a concentration of 100 g/L or more of sorbitol.
Liquid chromatography (2.2.29) as described in the test for related substances with the following modification.
Injection Test solution and reference solution (a).
Calculate the percentage content of d-sorbitol from the declared content of sorbitol CRS.
The label states:
- — where applicable, the maximum concentration of bacterial endotoxins;
- — where applicable, that the substance is suitable for use in the manufacture of parenteral preparations.
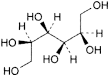
A. d-mannitol,
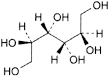
B. d-iditol,
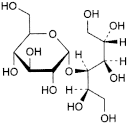
C. 4-O-α-d-glucopyranosyl-d-glucitol (d-maltitol).
This section provides information on characteristics that are recognised as being relevant control parameters for one or more functions of the substance when used as an excipient (see chapter 5.15). This section is a non-mandatory part of the monograph and it is not necessary to verify the characteristics to demonstrate compliance. Control of these characteristics can however contribute to the quality of a medicinal product by improving the consistency of the manufacturing process and the performance of the medicinal product during use. Where control methods are cited, they are recognised as being suitable for the purpose, but other methods can also be used. Wherever results for a particular characteristic are reported, the control method must be indicated.
The following characteristics may be relevant for sorbitol used as filler and binder in tablets.
Ph Eur