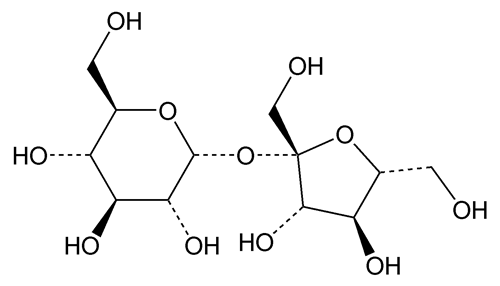
Sucrose
 • Residue on Ignition
• Residue on Ignition  281
281 :
NMT 0.05%, a 5-g specimen being used
:
NMT 0.05%, a 5-g specimen being used NF30
NF30
 • Chloride and Sulfate, Chloride
• Chloride and Sulfate, Chloride  221
221 :
A 2.0-g portion shows no more chloride than corresponds to 0.10 mL of 0.020 N hydrochloric acid (35 ppm).
:
A 2.0-g portion shows no more chloride than corresponds to 0.10 mL of 0.020 N hydrochloric acid (35 ppm). NF30
NF30
 • Chloride and Sulfate, Sulfate
• Chloride and Sulfate, Sulfate  221
221 :
A 5.0-g portion shows no more sulfate than corresponds to 0.30 mL of 0.020 N sulfuric acid (60 ppm).
:
A 5.0-g portion shows no more sulfate than corresponds to 0.30 mL of 0.020 N sulfuric acid (60 ppm). NF30
NF30
 • Calcium:
To 10 mL of a solution (1 in 10) add 1 mL of ammonium oxalate TS: the solution remains clear for at least 1 min.
• Calcium:
To 10 mL of a solution (1 in 10) add 1 mL of ammonium oxalate TS: the solution remains clear for at least 1 min. NF30
NF30
 • Heavy Metals
• Heavy Metals  231
231 :
Dissolve 4.0 g in 15 mL of water, add 1 mL of 0.12 N hydrochloric acid, and dilute with water to 25 mL.
:
Dissolve 4.0 g in 15 mL of water, add 1 mL of 0.12 N hydrochloric acid, and dilute with water to 25 mL.
 • Sulfite
• Sulfite
 • Appearance of Solution
• Appearance of Solution
 • Conductivity
• Conductivity
 781S
781S
 • Color Value
• Color Value
 • Dextrins
• Dextrins
 • Reducing Sugars
• Reducing Sugars
 • Loss on Drying
• Loss on Drying  731
731 :
Dry 2.000 g at 105
:
Dry 2.000 g at 105 for 3 h: it loses NMT 0.1% of its weight.
for 3 h: it loses NMT 0.1% of its weight. NF30
NF30
 • Bacterial Endotoxins
• Bacterial Endotoxins  85
85 :
Less than 0.25 IU/mg
:
Less than 0.25 IU/mg
 NF30
NF30
 • Invert Sugar
• Invert Sugar
 • Labeling:
The label states, where applicable, that the substance is suitable for use in the manufacture of large-volume parenteral dosage forms.
• Labeling:
The label states, where applicable, that the substance is suitable for use in the manufacture of large-volume parenteral dosage forms. NF30
NF30
(soo' krose).
Portions of the monograph text that are national USP text, and are not part of the harmonized text, are marked with symbols (
 ) to specify this fact.
) to specify this fact.
DEFINITION
Sucrose is a sugar obtained from Saccharum officinarum Linné (Fam. Gramineae), Beta vulgaris Linné (Fam. Chenopodiaceae), and other sources. It contains no added substances.
IMPURITIES
Delete the following:
Delete the following:
Delete the following:
Delete the following:
Delete the following:
Acceptance criteria:
NMT 5 ppm NF30
NF30
Add the following:
Sample solution:
400 mg/mL of Sucrose in freshly prepared distilled water
Sulfite standard solution (80 ppm SO2):
0.1575 mg/mL of anhydrous sodium sulfite in freshly prepared distilled water
Reference solution:
Dissolve 4.0 g of Sucrose in freshly prepared distilled water, add 0.5 mL of Sulfite standard solution (80 ppm SO2), and dilute with freshly prepared distilled water to 10.0 mL.
Blank:
Freshly prepared distilled water
Analysis:
Determine the sulfite content by a suitable enzymatic method based on the following reactions. Sulfite is oxidized by sulfite oxidase to sulfate and hydrogen peroxide which in turn is reduced by nicotinamide–adenine dinucleotide–peroxidase in the presence of reduced nicotinamide–adenine dinucleotide (NADH). The amount of NADH oxidized is proportional to the amount of sulfite. Separately introduce 2.0 mL each of the Sample solution, Reference solution, and Blank in 10-mm cuvettes, and add the reagents as described in the kit instructions. Measure the absorbance at the maximum at about 340 nm before and at the end of the reaction time, and subtract the value obtained with the Blank.
Acceptance criteria:
The absorbance difference of the Sample solution is not greater than half the absorbance difference of the Reference solution. NF30
NF30
SPECIFIC TESTS
Add the following:
Sample solution:
500 mg/mL of Sucrose in water. [Note—Set a portion of this solution aside for the tests for Dextrins and Reducing Sugars. ]
Hydrazine sulfate solution:
10 mg/mL of hydrazine sulfate in water. Allow to stand for 4–6 h.
Hexamethylenetetramine solution:
In a 100-mL ground-glass-stoppered flask, dissolve 2.5 g of hexamethylenetetramine in 25.0 mL of water.
Primary opalescent suspension:
To the Hexamethylenetetramine solution in the flask add 25.0 mL of Hydrazine sulfate solution. Mix, and allow to stand for 24 h. This suspension is stable for 2 months, provided it is stored in a glass container free from surface defects. The suspension must not adhere to the glass and must be well mixed before use.
Standard of opalescence:
Primary opalescent suspension in water (3 in 200). This suspension is freshly prepared and may be stored for up to 24 h.
Reference suspension I:
Standard of opalescence and water (5:95)
Acceptance criteria:
The clarity of the Sample solution is the same as that of water or its opalescence is not more pronounced than that of Reference suspension I. NF30
NF30
Add the following:
Sample solution:
313 mg/mL of Sucrose in freshly boiled and cooled water
Apparatus:
Use a conductivity meter or resistivity meter that measures the resistance of the column of liquid between the electrodes of the immersed measuring device. The apparatus is supplied with alternating current to avoid the effects of electrode polarization. It is equipped with a temperature compensation device or a precision thermometer.
Calibration:
Choose a conductivity cell that is appropriate for the conductivity of the solution to be examined. The higher the expected conductivity, the higher the cell constant that must be chosen so that the value measured, R, is as large as possible for the apparatus used. Commonly used conductivity cells have cell constants on the order of 0.1 cm 1, 1 cm
1, 1 cm 1, and 10 cm
1, and 10 cm 1. Use a standard solution of potassium chloride that is appropriate for the measurement. Rinse the cell several times with water that has been previously boiled and cooled to room temperature and at least twice with the potassium chloride solution used for the determination of the cell constant of the conductivity cell. Measure the resistance of the conductivity cell using the potassium chloride solution at 20 ± 0.1
1. Use a standard solution of potassium chloride that is appropriate for the measurement. Rinse the cell several times with water that has been previously boiled and cooled to room temperature and at least twice with the potassium chloride solution used for the determination of the cell constant of the conductivity cell. Measure the resistance of the conductivity cell using the potassium chloride solution at 20 ± 0.1 .
.
The constant, C (in cm 1), of the conductivity cell is given by the expression:
1), of the conductivity cell is given by the expression:
C = RKCl × KKCl
| RKCl | = | = measured resistance (M |
| KKCl | = | = conductivity of the standard solution of potassium chloride used (µS·cm |
The measured constant, C, of the conductivity cell must be within 5% of the given value.
Analysis
Sample:
Sample solution
Measure the conductivity of the Sample solution (C1), while gently stirring with a magnetic stirrer, and that of the water used for preparing the Sample solution (C2). The readings must be stable within 1% over a period of 30 s.
Calculate the conductivity of the Sample solution from the expression:
Result = C1  (0.35 × C2)
(0.35 × C2)
| C1 | = | = conductivity of the Sample solution |
| C2 | = | = water used for preparing the Sample solution |
Acceptance criteria:
NMT 35 µS·cm 1 at 20
1 at 20
 NF30
NF30
Change to read:
• Optical Rotation, Specific Rotation
Sample solution:
Previously dried Sucrose at 105 for 2 h. Prepare a solution of 260 mg/mL of Sucrose in water.
for 2 h. Prepare a solution of 260 mg/mL of Sucrose in water.
Acceptance criteria:
 +66.3 to +67.0
+66.3 to +67.0 NF30
NF30
Add the following:
Sample solution:
Dissolve 50.0 g in 50.0 mL of water. Mix, filter (diameter of pores, 0.45 µm), and degas.
Analysis:
Measure the absorbance at 420 nm, using a cell of at least 4 cm (a cell length of 10 cm or more is preferred).
Calculate the Color Value using the expression:
Result = (A × 1000)/(b × c)
| A | = | = absorbance measured at 420 nm |
| b | = | = cell path length (cm) |
| c | = | = concentration of the solution (g/mL), calculated from the refractive index of the solution. Use Table 1, and interpolate the values if necessary. The absolute difference between two results is NMT 3. |
Table 1
| n20D | c (g/mL) |
|---|---|
| 1.4138 | 0.570 |
| 1.4159 | 0.585 |
| 1.4179 | 0.600 |
| 1.4200 | 0.615 |
| 1.4221 | 0.630 |
| 1.4243 | 0.645 |
| 1.4264 | 0.661 |
Acceptance criteria:
NMT 45 NF30
NF30
Add the following:
[Note—If intended for use in the preparation of large-volume infusions, it complies with the test for Dextrins. ]
Sample solution:
Prepare as directed in the test for Appearance of Solution.
Analysis:
To 2 mL of the Sample solution add 8 mL of water, 0.05 mL of dilute hydrochloric acid (73 g/L of HCl), and 0.05 mL of 0.05 M iodine.
Acceptance criteria:
The solution remains yellow. NF30
NF30
Add the following:
Sample solution:
Prepare as directed in the test for Appearance of Solution.
Analysis:
To 5 mL of the Sample solution in a test tube, about 150 mm long and 16 mm in diameter, add 5 mL of water, 1.0 mL of 1 M sodium hydroxide, and 1.0 mL of a 1-g/L solution of methylene blue. Mix and place in a water bath. After exactly 2 min, take the tube out of the bath, and examine the solution immediately.
Acceptance criteria:
The blue color does not disappear completely, ignoring any blue color at the air/solution interface. NF30
NF30
Add the following:
Add the following:
[Note—If intended for use in the preparation of large-volume infusions, it complies with the test for Bacterial Endotoxins. ]
Delete the following:
Sample solution:
200 mg/mL of Sucrose in water
[Note—Filter if necessary. ]
Analysis:
Place 50 mL of the clear liquid in a 250-mL beaker, add 50 mL of alkaline cupric tartrate TS, cover the beaker with a watch glass, and heat the mixture at such a rate that it comes to a boil in approximately 4 min, and boil for 2 min, accurately timed. Add at once 100 mL of cold, recently boiled water, and immediately collect the precipitated cuprous oxide on a tared filtering crucible containing a sintered-glass disk of medium pore size, or suitable equivalent. Thoroughly wash the residue on the filter with hot water, then with 10 mL of alcohol, and finally with 10 mL of ether, and dry at 105 for 1 h.
for 1 h.
Acceptance criteria:
The weight of the cuprous oxide does not exceed 112 mg. NF30
NF30
ADDITIONAL REQUIREMENTS
•  Packaging and Storage:
Preserve in well-closed containers.
Packaging and Storage:
Preserve in well-closed containers.
Add the following:
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S.
Scientific Liaison 1-301-816-8335 |
(EXC2010) Monographs - Excipients |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology |
USP35–NF30 Page 1995
Pharmacopeial Forum: Volume No. 31(3) Page 902