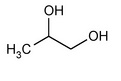Propylene Glycol
(proe' pi leen glye' kol).
DEFINITION
Propylene Glycol contains NLT 99.5% of C3H8O2.
IDENTIFICATION
[Note—Compliance is determined by meeting the requirements of Identification tests A, B, and C. ]
• A. Infrared Absorption  197F
197F
[Note—Undried specimen. ]
• B. Limit of Diethylene Glycol and Ethylene Glycol
Diluent:
Methanol
Standard solution:
2.0 mg/mL of USP Propylene Glycol RS, 0.050 mg/mL of USP Ethylene Glycol RS, 0.050 mg/mL of USP Diethylene Glycol RS, and 0.10 mg/mL of 2,2,2-trichloroethanol (internal standard) in methanol
Sample solution:
50 mg/mL of Propylene Glycol and 0.10 mg/mL of 2,2,2-trichloroethanol (internal standard) in methanol
Chromatographic system
Mode:
GC
Detector:
Flame ionization
Column:
0.53-mm × 30-m fused-silica coated with 3.0-µm G43 stationary phase, and a deactivated split liner with glass wool
Temperature
Injector:
220
Detector:
250
Column:
See the temperature program table below.
| Initial Temperature ( |
Temperature Ramp ( |
Final Temperature ( |
Hold Time at Final Temperature (min) |
|---|---|---|---|
| 100 | — | 100 | 4 |
| 100 | 50 | 120 | 10 |
| 120 | 50 | 220 | 6 |
Carrier gas:
Helium
Injection size:
1.0 µL
Flow rate:
4.5 mL/min
Injection type:
The split flow ratio is about 10:1.
System suitability
Sample:
Standard solution
[Note—For informational purposes only. See Impurity Table 1 for relative retention times for ethylene glycol, internal standard, and diethylene glycol. The retention time for propylene glycol is 4 min. ]
Impurity Table 1
| Component | Relative Retention Time |
|---|---|
| Ethylene glycol | 0.8 |
| Propylene glycol | 1.0 |
| Internal standard | 1.7 |
| Diethylene glycol | 2.4 |
Suitability requirements
Resolution:
NLT 5 between ethylene glycol and propylene glycol
Analysis
Sample:
Sample solution
Acceptance criteria
Diethylene glycol:
If a peak at the retention time for diethylene glycol is present in the Sample solution, the peak response ratio relative to 2,2,2-trichloroethanol is NMT the peak response ratio for diethylene glycol relative to 2,2,2-trichloroethanol in the Standard solution: NMT 0.10% for diethylene glycol.
Ethylene glycol:
If a peak at the retention times for ethylene glycol is present in the Sample solution, the peak response ratio relative to 2,2,2-trichloroethanol is not more than the peak response ratio for ethylene glycol relative to 2,2,2-trichloroethanol in the Standard solution: NMT 0.10% for ethylene glycol is found.
• C.
Examine the chromatograms obtained in Identification test B. The retention time of the propylene glycol peak of the Sample solution corresponds to that of the Standard solution.
ASSAY
• Procedure
Sample:
Propylene Glycol
Chromatographic system
Mode:
GC
Detector:
Thermal conductivity
Column:
1-m × 4-mm; 5% phase G16; support S5
Temperature
Injector:
240
Detector:
250
Column:
Increase from 120 to 200
to 200 at a rate of5
at a rate of5 /min.
/min.
Carrier gas:
Helium
Injection size:
10 µL
[Note—The approximate retention time for propylene glycol is 5.7 min, and the approximate retention times for the three isomers of dipropylene glycol, when present, are 8.2, 9.0, and 10.2 min, respectively. ]
Analysis:
Calculate the percentage of C3H8O2 in the Sample by dividing the area under the propylene glycol peak by the sum of the areas under all of the peaks, excluding those due to air and water, and multiplying by 100:
Result = [rU/(rU + SrU)] × 100
| rU | = | = peak response for Propylene Glycol from the Sample |
| SrU | = | = sum of individual impurity peak responses, excluding those due to air and water, from the Sample |
Acceptance criteria:
NLT 99.5%
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281
Sample:
50 g
Analysis:
Heat the Sample in a tared 100-mL shallow dish until it ignites, and allow it to burn without further application of heat in a place free from drafts. Cool, moisten the residue with 0.5 mL of sulfuric acid, and ignite to constant weight.
Acceptance criteria:
The weight of the residue is NMT 3.5 mg.
• Chloride and Sulfate, Chloride  221
221 :
A 1-mL portion shows no more chloride than corresponds to 0.10 mL of 0.020 N hydrochloric acid (70 ppm).
:
A 1-mL portion shows no more chloride than corresponds to 0.10 mL of 0.020 N hydrochloric acid (70 ppm).
• Chloride and Sulfate, Sulfate  221
221 :
A 5.0-mL portion shows no more sulfate than corresponds to 0.30 mL of 0.020 N sulfuric acid (60 ppm).
:
A 5.0-mL portion shows no more sulfate than corresponds to 0.30 mL of 0.020 N sulfuric acid (60 ppm).
SPECIFIC TESTS
• Specific Gravity  841
841 :
1.035–1.037
:
1.035–1.037
• Acidity
Sample:
10 mL of Propylene Glycol
Analysis:
Add 1 mL of phenolphthalein TS to 50 mL of water, then add 0.1 N sodium hydroxide until the solution remains pink for 30 s. Add the Sample, and titrate with 0.10 N sodium hydroxide until the original pink color returns and remains for 30 s.
Acceptance criteria:
NMT 0.20 mL of 0.10 N sodium hydroxide
• Water Determination, Method I  921
921 :
NMT 0.2%
:
NMT 0.2%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers.
• USP Reference Standards  11
11
USP Ethylene Glycol RS
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S.
Scientific Liaison 1-301-816-8335 |
(EXC2010) Monographs - Excipients |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4469
Pharmacopeial Forum: Volume No. 33(2) Page 317