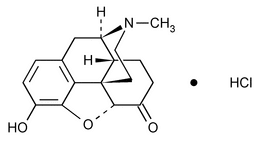
Hydromorphone Hydrochloride
C17H19NO3·HCl 321.80
Morphinan-6-one, 4,5-epoxy-3-hydroxy-17-methyl-, hydrochloride, (5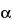 )-;
)-;
4,5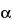 -Epoxy-3-hydroxy-17-methylmorphinan-6-one hydrochloride
-Epoxy-3-hydroxy-17-methylmorphinan-6-one hydrochloride


 [71-68-1].
[71-68-1].

 USP35
USP35
(hye'' droe mor' fone hye'' droe klor' ide).
C17H19NO3·HCl 321.80
Morphinan-6-one, 4,5-epoxy-3-hydroxy-17-methyl-, hydrochloride, (5
4,5
DEFINITION
Hydromorphone Hydrochloride, dried at 105 for 2 h, contains NLT 98.0% and NMT 101.0% of C17H19NO3·HCl.
for 2 h, contains NLT 98.0% and NMT 101.0% of C17H19NO3·HCl.
IDENTIFICATION
• B. Ultraviolet Absorption  197U
197U
Sample solution:
100 µg/mL
Analytical wavelength:
280 nm
Acceptance criteria:
Absorptivities, calculated on the dried basis, do not differ by more than 3.0%.
• C. Identification Tests—General, Chloride  191
191
Sample solution:
1 in 20
Acceptance criteria:
Meets the requirements
ASSAY
• Procedure
Sample:
225 mg, previously dried
Analysis:
Transfer the Sample to a 250-mL conical flask. Dissolve in 80 mL of glacial acetic acid, warming, if necessary. Cool, and add 5 mL of acetic anhydride and 10 mL of mercuric acetate TS. Add 1 drop of crystal violet TS, and titrate with 0.1 N perchloric acid VS to a blue endpoint. Perform a blank determination, and make any necessary correction (see Titrimetry  541
541 ). Each mL of 0.1 N perchloric acid is equivalent to 32.18 mg of C17H19NO3·HCl.
). Each mL of 0.1 N perchloric acid is equivalent to 32.18 mg of C17H19NO3·HCl.
Acceptance criteria:
98.0%–101.0%, dried at 105 for 2 h
for 2 h
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.3%
:
NMT 0.3%
• Sulfate
Sample solution:
100 mg in 5 mL of water
Analysis:
To the Sample solution add 0.5 mL of 3 N hydrochloric acid and 1 mL of barium chloride TS.
Acceptance criteria:
No turbidity is produced.
Change to read:
Organic Impurities
[Note—If (5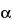 )-7-[(5
)-7-[(5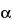 )-3,6-dihydroxy-17-methyl-4,5-epoxymorphinan-6-yl]-3-hydroxy-17-methyl-4,5-epoxymorphinan-6-one (hydromorphone aldol dimer) or (5
)-3,6-dihydroxy-17-methyl-4,5-epoxymorphinan-6-yl]-3-hydroxy-17-methyl-4,5-epoxymorphinan-6-one (hydromorphone aldol dimer) or (5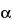 )-3-hydroxy-8-[(5
)-3-hydroxy-8-[(5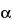 )-3-hydroxy-17-methyl-6-oxo-4,5-epoxymorphinan-7-yl]-17-methyl-4,5-epoxymorphinan-6-one (7,8'-bishydromorphone) are potential impurities, Procedure 2 is recommended. ]
)-3-hydroxy-17-methyl-6-oxo-4,5-epoxymorphinan-7-yl]-17-methyl-4,5-epoxymorphinan-6-one (7,8'-bishydromorphone) are potential impurities, Procedure 2 is recommended. ]
• Procedure 1
Diluent:
Phosphoric acid and water (1:1000)
Solution A:
1.0 mg/mL of sodium 1-heptanesulfonate monohydrate in 1000 mL of methanol and water (1:9). Add 1.0 mL of triethylamine, and adjust with phosphoric acid to a pH of 2.5 ± 0.1.
Solution B:
1.0 mg/mL of sodium 1-heptanesulfonate monohydrate in 1000 mL of methanol and water (1:1). Add 1.0 mL of triethylamine, and adjust with phosphoric acid to a pH of 2.5 ± 0.1.
Mobile phase:
See the gradient table below.
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 94 | 6 |
| 25 | 94 | 6 |
| 40 | 20 | 80 |
| 70 | 20 | 80 |
| 75 | 94 | 6 |
| 90 | 94 | 6 |
System suitability solution:
0.8 mg/mL each of USP Hydromorphone Hydrochloride RS and USP Hydromorphone Related Compound A RS in Diluent. [Note—The solution should be kept in a cool place protected from light. ]
Standard solution:
4 µg/mL of USP Hydromorphone Hydrochloride RS in Diluent
Sample solution:
0.8 mg/mL of Hydromorphone Hydrochloride in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 220 nm
Column:
3.9-mm × 15-cm; 5-µm packing L1
Column temperature:
45
Flow rate:
1.0 mL/min
Injection size:
20 µL
System suitability
Samples:
System suitability solution and Standard solution
Suitability requirements
Resolution:
NLT 1.0 between the hydromorphone related compound A and hydromorphone peaks, System suitability solution
Tailing factor:
NMT 1.5 for the hydromorphone peak, Standard solution
Relative standard deviation:
NMT 5.0%, Standard solution
Analysis
Samples:
Diluent, Standard solution, and Sample solution
Calculate the percentage of any specified or unspecified impurity in the portion of Hydromorphone Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 1/F × 100
| rU | = | = peak response for each degradation found, including those in Impurity Table 1, from the Sample solution |
| rS | = | = peak response of hydromorphone from the Standard solution |
| CS | = | = concentration of USP Hydromorphone Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Hydromorphone Hydrochloride in the Sample solution (mg/mL) |
| F | = | = relative response factor for the corresponding individual specified or unspecified impurity from Impurity Table 1 |
Acceptance criteria:
See Impurity Table 1. [Note—Disregard peaks corresponding to those obtained from the Diluent. ]
Impurity Table 1
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| 8-Hydroxyhydromorphonea | 0.50 | 1.0 | 0.15 |
| Dihydromorphine (DHM)b | 0.61 | 1.0 | 0.5 |
| Morphinec | 0.65 | 1.8 | 0.15 |
| Hydromorphone N-oxided |
0.79 | 1.0 | 0.15 |
| Hydromorphone related compound Ae |
0.93 | 1.4 | 0.1 |
| Hydromorphone | 1.0 | — | — |
| 8,14-Dihydrooripavine* | 1.66 | 1.0 | 0.15 |
| 6 |
1.71 | 1.0 | 0.15 |
| 2,2'-Bis hydromorphonef | 2.02 | 1.7 | 0.15 |
| Individual unspecified impurities | — | 1.0 | 0.1 |
| Total impurities | — | — | 1.0 |
|
a
4,5
b
4,5
c
7,8-Didehydro-4,5
d
4,5
e
7,8-Didehydro-4,5
f
(5
*
8,14-Dihydrooripavine and 6
|
|||
• Procedure 2
Diluent:
Phosphoric acid and water (1:100)
Solution A:
Mix 3520 mL of water, 18.40 g of monobasic ammonium phosphate, and 4.32 g of sodium 1-octanesulfonate. Add 4.0 mL of triethylamine, adjust with phosphoric acid to a pH of 2.90, and add 480 mL of acetonitrile.
Solution B:
Acetonitrile and water (1600:400)
Mobile phase:
See the gradient table below.
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 100 | 0 |
| 20 | 80 | 20 |
| 21 | 100 | 0 |
| 30 | 100 | 0 |
System suitability solution:
0.15 mg/mL of USP Hydromorphone Hydrochloride RS and 0.1 mg/mL of USP Morphine RS in Diluent. [Note—The solution should be kept in a cool place protected from light. ]
Standard solution:
15 µg/mL of USP Hydromorphone Hydrochloride RS in Diluent
Sample solution:
3 mg/mL of Hydromorphone Hydrochloride in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 280 nm
Column:
3.9-mm × 15-cm; 5-µm packing L7
Column temperature:
40
Flow rate:
1.5 mL/min
Injection size:
10 µL
Run time:
30 min
System suitability
Samples:
System suitability solution and Standard solution
Suitability requirements
Resolution:
NLT 4.0 between the morphine and hydromorphone peaks, System suitability solution
Tailing factor:
NMT 1.5 for the hydromorphone peak, System suitability solution
Relative standard deviation:
NMT 5.0%, Standard solution
Analysis
Samples:
Diluent, Standard solution, and Sample solution
Calculate the percentage of any specified or unspecified impurity in the portion of Hydromorphone Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 1/F × 100
| rU | = | = peak response for each peak found, including those in Impurity Table 2, from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Hydromorphone Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Hydromorphone Hydrochloride in the Sample solution (mg/mL) |
| F | = | = relative response factor for the corresponding individual specified or unspecified impurity from Impurity Table 2 |
Acceptance criteria:
See Impurity Table 2. [Note—Disregard peaks corresponding to those obtained from the Diluent. ]
Impurity Table 2
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Dihydromorphine (DHM)a | 0.63 | 1.0 | 0.5 |
| Morphineb | 0.70 | 1.0 | 0.5 |
| Hydromorphone | 1.0 | — | — |
| 2,2'-Bishydromorphonec | 1.82 | 2.0 | 0.5 |
| Hydromorphone Hydrochloride Aldol Dimerd | 2.12 | 1.0 | 0.5 |
| 7,8-Bishydromorphonee | 2.32 | 1.0 | 0.5 |
| Individual unspecified impurities | — | 1.0 | 0.10 |
| Total impurities | — | — | 2.0 |
|
a
4,5
b
7,8-Didehydro-4,5
c
(5
d
(5
e
(5
|
|||
SPECIFIC TESTS
• Optical Rotation, Specific Rotation  781S
781S
Sample solution:
50 mg/mL
Acceptance criteria:
Between  136
136 and
and  139
139
• Acidity
Sample:
300 mg
Analysis:
Dissolve the Sample in 10 mL of water, add 1 drop of methyl red TS, and titrate with 0.020 N sodium hydroxide VS.
Acceptance criteria:
NMT 0.30 mL is required to produce a yellow color.
• Loss on Drying  731
731 :
Dry a sample at 105
:
Dry a sample at 105 for 2 h: it loses NMT 1.5% of its weight.
for 2 h: it loses NMT 1.5% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers. Store at 25 , excursions permitted between 15
, excursions permitted between 15 and 30
and 30 .
.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Clydewyn M. Anthony, Ph.D.
Senior Scientific Liaison 1-301-816-8139 |
(SM22010) Monographs - Small Molecules 2 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3446
Pharmacopeial Forum: Volume No. 35(5) Page 1156