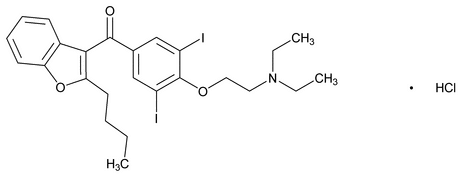
Amiodarone Hydrochloride
C25H29I2NO3·HCl 681.77
Methanone, (2-butyl-3-benzofuranyl)[4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]- hydrochloride;
2-Butyl-3-benzofuranyl 4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl ketone hydrochloride

 [19774-82-4].
[19774-82-4].
2-Butyl-3-benzofuranyl 4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl ketone

 [1951-25-3].
[1951-25-3].
C25H29I2NO3·HCl 681.77
Methanone, (2-butyl-3-benzofuranyl)[4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]- hydrochloride;
2-Butyl-3-benzofuranyl 4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl ketone hydrochloride
2-Butyl-3-benzofuranyl 4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl ketone
DEFINITION
Amiodarone Hydrochloride contains NLT 98.5% and NMT 101.0% of C25H29I2NO3·HCl, calculated on the dried basis.
IDENTIFICATION
• B. Identification Tests—General, Chloride  191
191 :
Meets the requirements
:
Meets the requirements
ASSAY
• Procedure
Buffer:
Dissolve 6.80 g of monobasic potassium phosphate in 900 mL of water, and add 1.0 mL of triethylamine. Adjust with phosphoric acid to a pH of 6.00 ± 0.05, and dilute with water to 1000 mL.
Diluent:
Acetonitrile and water (1:1)
Mobile phase:
Acetonitrile and Buffer (1:1)
Standard stock solution:
0.5 mg/mL of USP Amiodarone Hydrochloride RS in methanol
Standard solution:
0.1 mg/mL USP Amiodarone Hydrochloride RS in Diluent from Standard stock solution
Sample stock solution:
0.5 mg/mL of Amiodarone Hydrochloride in methanol
Sample solution:
0.1 mg/mL of Amiodarone Hydrochloride in Diluent from Sample stock solution
Chromatographic system
Mode:
LC
Detector:
UV 240 nm
Column:
3.9-mm × 15-cm; 5-µm packing L26
Flow rate:
1.5 mL/min
Injection size:
10 µL
System suitability
Sample:
Standard solution
Suitability requirements
Column efficiency:
NLT 1000 theoretical plates
Tailing factor:
NMT 2.0
Relative standard deviation:
NMT 1.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C25H29I2NO3·HCl in the portion of Amiodarone Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of amiodarone in the Sample solution |
| rS | = | = peak response of amiodarone in the Standard solution |
| CS | = | = concentration of USP Amiodarone Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of Amiodarone Hydrochloride in the Sample solution (mg/mL) |
Acceptance criteria:
98.5%–101.0%, on the dried basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.1% on a 1-g sample
:
NMT 0.1% on a 1-g sample
• Heavy Metals
Buffer:
Dissolve 25.0 g of ammonium acetate in 25 mL of water, and add 38.0 mL of 70% hydrochloric acid. Adjust, if necessary, with diluted hydrochloric acid or diluted ammonia solution to a pH of 3.5. Dilute with water to 100.0 mL.
Lead standard stock solution (1000 ppm Pb):
1.6 mg/mL of lead nitrate in water
Lead standard solution:
10 ppm of lead in water from Lead standard stock solution. [Note—Prepare immediately before use. ]
Phenolphthalein solution:
Dissolve 0.1 g of phenolphthalein in 80 mL of alcohol, and dilute with water to 100 mL.
Thioacetamide solution:
Prepare a solution of 40 g/L of thioacetamide in water. To 0.2 mL of the freshly prepared solution, add 1 mL of a mixture of 85% glycerol, 1 M sodium hydroxide, and water (4:3:1). Heat in a water bath for 20 s.
Sample solution:
Place about 1 g of Amiodarone Hydrochloride in a silica crucible along with 4 mL of magnesium sulfate solution (250 g/L of diluted sulfuric acid). Mix using a fine glass rod, and heat cautiously. If the mixture is a liquid, evaporate gently to dryness on a water bath. Progressively heat to ignition, and continue heating until an almost white or a mostly grayish residue is obtained. Carry out the ignition at a temperature not exceeding 800 . Allow to cool. Moisten the residue with a few drops of dilute sulfuric acid. Evaporate, ignite again, and allow to cool. The total period of ignition must not exceed 2 h. Dissolve the residue in two portions, 5 mL each, of 20% hydrochloric acid. Add 0.1 mL of Phenolphthalein solution followed by 25% ammonia water until a pink color is obtained. Cool, add glacial acetic acid until the solution is decolorized, and add 0.5 mL in excess. Filter if necessary, wash the filter, and dilute with water to 20 mL.
. Allow to cool. Moisten the residue with a few drops of dilute sulfuric acid. Evaporate, ignite again, and allow to cool. The total period of ignition must not exceed 2 h. Dissolve the residue in two portions, 5 mL each, of 20% hydrochloric acid. Add 0.1 mL of Phenolphthalein solution followed by 25% ammonia water until a pink color is obtained. Cool, add glacial acetic acid until the solution is decolorized, and add 0.5 mL in excess. Filter if necessary, wash the filter, and dilute with water to 20 mL.
Standard solution:
Proceed as directed for Sample solution, using 2 mL of Lead standard solution instead of Amiodarone Hydrochloride. To 10 mL of the solution obtained, add 2 mL of the Sample solution.
Monitor solution:
Proceed as directed for Sample solution, adding 2 mL of Standard solution to 1 g of Amiodarone Hydrochloride.
Blank solution:
10 mL of water and 2 mL of Sample solution
Analysis
Samples:
Standard solution, Sample solution, Blank solution, and Monitor solution
To 12 mL each of the Standard solution, Sample solution, Blank solution, and Monitor solution add 2 mL of Buffer solution, and mix. Add 1.2 mL of Thioacetamide solution, and immediately mix again. Examine the solutions after 2 min. The test is invalid if the Standard solution does not show a slight brown color compared to the Blank solution or if the Monitor solution is not comparable with the Standard solution.
Acceptance criteria:
Any brown color in the Sample solution is not more intense than that in the Standard solution (20 ppm). [Note—If the result is difficult to judge, pass the solutions through a membrane filter having a porosity of 3 µm. Carry out the filtration slowly and uniformly, applying moderate and constant pressure. Compare the spots on the filters obtained from the different solutions. ]
Organic Impurities
[Note—The product meets the requirements for both Procedure 1 and Procedure 2. ]
• Procedure 1
Potassium iodobismuthate solution:
Dissolve 100 g of tartaric acid in 400 mL of water, and add 8.5 g of bismuth subnitrate. Shake for 1 h, add 200 mL of a 400 g/L solution of potassium iodide, and shake well. Allow to stand for 24 h, filter, and protect from light.
Standard solution A:
0.02 mg/mL of USP Amiodarone Related Compound H RS in methylene chloride
Standard solution B:
Standard solution A and Sample solution (1:1).
Sample solution:
100 mg/mL of Amiodarone Hydrochloride in methylene chloride
Chromatographic system
Mode:
TLC
Adsorbent:
0.5-mm layer of chromatographic silica gel and fluorescent indicator with maximum absorbance at 254 nm
Application volume
Standard solution A:
50 µL
Standard solution B:
100 µL
Sample solution:
50 µL
Developing solvent system:
Methylene chloride, methanol, and anhydrous formic acid (17:2:1)
Analysis
Samples:
Standard solution A, Standard solution B, and Sample solution
Develop the plate in the Developing solvent system until the solvent front has moved NLT two-thirds the length of the plate, and dry in a current of cold air. Spray the plate with Potassium iodobismuthate solution and then with 3% hydrogen peroxide solution. Examine immediately in daylight: the spot from Standard solution B due to amiodarone related compound H is clearly visible.
Acceptance criteria:
Any spot with the same RF as the spot due to amiodarone related compound H from Standard solution B is not more intense than the spot from Standard solution A (0.02%).
• Procedure 2
Buffer:
Add 3 mL of glacial acetic acid to 800 mL of water. Adjust with diluted ammonia solution to a pH of 4.9, and dilute with water to 1000 mL.
Mobile phase:
Acetonitrile: methanol: Buffer (4:3:3 v/v/v).
Diluent:
Acetonitrile and water (1:1)
Standard stock solution:
Dissolve equal quantities of USP Amiodarone Related Compound D RS, USP Amiodarone Related Compound E RS, and USP Amiodarone Hydrochloride RS in a known amount of methanol.
Standard solution:
0.01 mg/mL each of USP Amiodarone Related Compound D RS, USP Amiodarone Related Compound E RS, and USP Amiodarone Hydrochloride RS, in Diluent from Standard stock solution
Sample solution:
5 mg/mL of Amiodarone Hydrochloride in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 240 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Column temperature:
30
Flow rate:
1 mL/min
Injection size:
10 µL
Run time:
2 times the retention time of amiodarone
System suitability
Sample:
Standard solution
Suitability requirements
Resolution:
NLT 3.5 between amiodarone related compound D and amiodarone related compound E
Analysis
[Note—Disregard any peak that is less than 0.05%. ]
Samples:
Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Amiodarone Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of each impurity in the Sample solution |
| rS | = | = peak response of amiodarone in the Standard solution |
| CS | = | = concentration of USP Amiodarone Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of Amiodarone Hydrochloride in the Sample solution (mg/mL) |
Acceptance criteria
Individual impurities:
See Impurity Table I.
Total impurities:
NMT 0.5%
Impurity Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
||
|---|---|---|---|---|
| Amiodarone related compound Aa | 0.26 | 0.2 | ||
| Amiodarone related compound Db | 0.29 | 0.2 | ||
| Amiodarone related compound Ec | 0.37 | 0.2 | ||
| Amiodarone related compound Bd | 0.49 | 0.2 | ||
| Amiodarone related compound Ce | 0.55 | 0.2 | ||
| Amiodarone related compound Gf | 0.62 | 0.2 | ||
| Amiodarone related compound Fg | 0.69 | 0.2 | ||
| Amiodarone hydrochloride | 1.00 | — | ||
| Any other individual impurity | — | 0.10 | ||
|
a
(2-Butylbenzofuran-3-yl){4-[2-(diethylamino)ethoxy]phenyl}methanone.
b
(2-Butylbenzofuran-3-yl)(4-hydroxy-3,5-diiodophenyl)methanone.
c
(2-Butylbenzofuran-3-yl)(4-hydroxyphenyl)methanone.
d
(2-Butylbenzofuran-3-yl){4-[2-(ethylamino)ethoxy]-3,5-diiodophenyl}methanone.
e
(2-Butylbenzofuran-3-yl){4-[2-(diethylamino)ethoxy]-3-iodophenyl}methanone.
f
[2-[(1RS)-1-Methoxybutyl]benzofuran-3-yl][4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]methanone.
g
(2-Butylbenzofuran-3-yl)(4-hydroxy-3-iodophenyl)methanone.
|
||||
SPECIFIC TESTS
• Limit of Iodides
Solution A:
Add 1.50 g of Amiodarone Hydrochloride to 40 mL of water at 80 , and shake until completely dissolved. Cool, and dilute with water to 50.0 mL.
, and shake until completely dissolved. Cool, and dilute with water to 50.0 mL.
Standard solution:
To 15.0 mL of Solution A add 1.0 mL of 0.1 M hydrochloric acid, 1.0 mL of an 88.2 mg/L solution of potassium iodide, and 1.0 mL of 0.05 M potassium iodate. Dilute with water to 20.0 mL. Allow to stand protected from light for 4 h.
Sample solution:
To 15.0 mL of Solution A add 1.0 mL of 0.1 M hydrochloric acid and 1.0 mL of 0.05 M potassium iodate. Dilute with water to 20.0 mL. Allow to stand protected from light for 4 h.
Analysis:
Measure the absorbances of the Standard solution and the Sample solution at 420 nm, using a mixture of 15.0 mL of Solution A and 1.0 mL of 0.1 M hydrochloric acid diluted with water to 20.0 mL to serve as the blank. The absorbance of the Sample solution is NMT half the absorbance of the Standard solution.
Acceptance criteria:
NMT 150 ppm
• pH  791
791 :
3.2–3.8. Dissolve 1 g of Amiodarone Hydrochloride in water by heating at 80
:
3.2–3.8. Dissolve 1 g of Amiodarone Hydrochloride in water by heating at 80 . Cool, and dilute with water to 20 mL.
. Cool, and dilute with water to 20 mL.
• Loss on Drying  731
731 :
Use 1 g of sample, and dry under vacuum (NMT 0.3 kPa) at 50
:
Use 1 g of sample, and dry under vacuum (NMT 0.3 kPa) at 50 for 4 h: it loses NMT 0.5% of its weight.
for 4 h: it loses NMT 0.5% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in light-resistant, tight containers. Store at controlled room temperature.
• USP Reference Standards  11
11
USP Amiodarone Related Compound D RS 
(2-Butylbenzofuran-3-yl)(4-hydroxy-3,5-diiodophenyl) methanone.
C19H16I2O3 546.14

(2-Butylbenzofuran-3-yl)(4-hydroxy-3,5-diiodophenyl) methanone.
C19H16I2O3 546.14
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Sujatha Ramakrishna, Ph.D.
Senior Scientific Liaison 1-301-816-8349 |
(SM22010) Monographs - Small Molecules 2 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2180
Pharmacopeial Forum: Volume No. 34(6) Page 1429